Osteopontin in systemic sclerosis and its role in dermal fibrosis
- PMID: 22402440
- PMCID: PMC3365548
- DOI: 10.1038/jid.2012.32
Osteopontin in systemic sclerosis and its role in dermal fibrosis
Abstract
Osteopontin (OPN) is a matricellular protein with proinflammatory and profibrotic properties. Previous reports demonstrate a role for OPN in wound healing and pulmonary fibrosis. Here, we determined whether OPN levels are increased in a large cohort of patients with systemic sclerosis (SSc) and whether OPN contributes to the development of dermal fibrosis. The plasma OPN levels were increased in SSc patients, including patients with limited and diffuse disease, compared with healthy controls. Immunohistology demonstrated OPN on fibroblast-like and inflammatory cells in SSc skin and lesional skin from mice in the bleomycin (bleo)-induced dermal fibrosis model. OPN-deficient (OPN(-/-)) mice developed less dermal fibrosis compared with wild-type (WT) mice in the bleo-induced dermal fibrosis model. Additional in vivo studies have demonstrated that lesional skin from OPN(-/-)mice had fewer Mac-3-positive cells, fewer myofibroblasts, decreased transforming growth factor (TGF)-β and genes in the TGF-β pathway, and decreased numbers of cells expressing phosphorylated SMAD2 (pSMAD) and extracellular signal-regulated kinase. In vitro, OPN(-/-) dermal fibroblasts had decreased migratory capacity but similar phosphorylation of SMAD2 by TGF-β. Finally, TGF-β production by OPN-deficient macrophages was reduced compared with WT. These data demonstrate an important role for OPN in the development of dermal fibrosis and suggest that it may be a new therapeutic target in SSc.
Conflict of interest statement
Conflict of Interest: The authors declare no conflicts of interest.
Figures

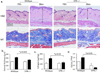



References
-
- Abraham DJ, Varga J. Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 2005;26:587–595. - PubMed
-
- Arnett FC. Is scleroderma an autoantibody mediated disease? Curr Opin Rheumatol. 2006;18:579–581. - PubMed
-
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR062056/AR/NIAMS NIH HHS/United States
- T32 AR052283/AR/NIAMS NIH HHS/United States
- NIH/NIAMS-K08AR054404/AR/NIAMS NIH HHS/United States
- P50 AR054144/AR/NIAMS NIH HHS/United States
- 5T32AR052283-03/AR/NIAMS NIH HHS/United States
- UL1 RR024148/RR/NCRR NIH HHS/United States
- N01-AR-0-2251/AR/NIAMS NIH HHS/United States
- P50AR054144/AR/NIAMS NIH HHS/United States
- KL2 RR024149/RR/NCRR NIH HHS/United States
- TL1 RR024147/RR/NCRR NIH HHS/United States
- R01 HL070952/HL/NHLBI NIH HHS/United States
- K08 AR054404/AR/NIAMS NIH HHS/United States
- NIH/NHLBI RO1HL70952-09/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

