Fabrication of an inexpensive, implantable cooling device for reversible brain deactivation in animals ranging from rodents to primates
- PMID: 22402651
- PMCID: PMC3378414
- DOI: 10.1152/jn.01101.2011
Fabrication of an inexpensive, implantable cooling device for reversible brain deactivation in animals ranging from rodents to primates
Abstract
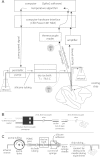
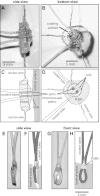
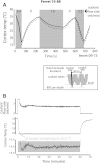
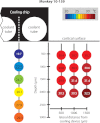
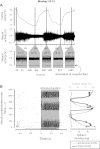
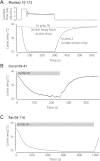
We have developed a compact and lightweight microfluidic cooling device to reversibly deactivate one or more areas of the neocortex to examine its functional macrocircuitry as well as behavioral and cortical plasticity. The device, which we term the "cooling chip," consists of thin silicone tubing (through which chilled ethanol is circulated) embedded in mechanically compliant polydimethylsiloxane (PDMS). PDMS is tailored to compact device dimensions (as small as 21 mm(3)) that precisely accommodate the geometry of the targeted cortical area. The biocompatible design makes it suitable for both acute preparations and chronic implantation for long-term behavioral studies. The cooling chip accommodates an in-cortex microthermocouple measuring local cortical temperature. A microelectrode may be used to record simultaneous neural responses at the same location. Cortex temperature is controlled by computer regulation of the coolant flow, which can achieve a localized cortical temperature drop from 37 to 20°C in less than 3 min and maintain target temperature to within ±0.3°C indefinitely. Here we describe cooling chip fabrication and performance in mediating cessation of neural signaling in acute preparations of rodents, ferrets, and primates.
Figures







Similar articles
-
Use of a Peltier chip with a newly devised local brain-cooling system for neocortical seizures in the rat. Technical note.J Neurosurg. 2006 Jan;104(1):150-6. doi: 10.3171/jns.2006.104.1.150. J Neurosurg. 2006. PMID: 16509160
-
Temperatures achieved in human and canine neocortex during intraoperative passive or active focal cooling.Ther Hypothermia Temp Manag. 2015 Jun;5(2):95-103. doi: 10.1089/ther.2014.0025. Epub 2015 Apr 22. Ther Hypothermia Temp Manag. 2015. PMID: 25902001 Free PMC article.
-
Brain temperature profiles during epidural cooling with the ChillerPad in a monkey model of traumatic brain injury.J Neurotrauma. 2010 Oct;27(10):1895-903. doi: 10.1089/neu.2009.1178. Epub 2010 Sep 16. J Neurotrauma. 2010. PMID: 20684677
-
The therapeutic potential of focal cooling for neocortical epilepsy.Neurotherapeutics. 2009 Apr;6(2):251-7. doi: 10.1016/j.nurt.2008.12.002. Neurotherapeutics. 2009. PMID: 19332317 Free PMC article. Review.
-
Application of focal cerebral cooling for the treatment of intractable epilepsy.Neurol Med Chir (Tokyo). 2010;50(9):839-44. doi: 10.2176/nmc.50.839. Neurol Med Chir (Tokyo). 2010. PMID: 20885118 Review.
Cited by
-
Reversible deactivation of higher-order posterior parietal areas. II. Alterations in response properties of neurons in areas 1 and 2.J Neurophysiol. 2014 Nov 15;112(10):2545-60. doi: 10.1152/jn.00141.2014. Epub 2014 Aug 20. J Neurophysiol. 2014. PMID: 25143537 Free PMC article.
-
Cytostatic hypothermia and its impact on glioblastoma and survival.Sci Adv. 2022 Nov 25;8(47):eabq4882. doi: 10.1126/sciadv.abq4882. Epub 2022 Nov 25. Sci Adv. 2022. PMID: 36427309 Free PMC article.
-
Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures.Adv Mater. 2013 Oct 11;25(38):5477-82. doi: 10.1002/adma.201302219. Epub 2013 Jul 4. Adv Mater. 2013. PMID: 23824715 Free PMC article.
-
Focal Cooling for Drug-Resistant Epilepsy: A Review.JAMA Neurol. 2022 Sep 1;79(9):937-944. doi: 10.1001/jamaneurol.2022.1936. JAMA Neurol. 2022. PMID: 35877102 Free PMC article. Review.
-
Reversible Deactivation of Motor Cortex Reveals Functional Connectivity with Posterior Parietal Cortex in the Prosimian Galago (Otolemur garnettii).J Neurosci. 2015 Oct 21;35(42):14406-22. doi: 10.1523/JNEUROSCI.1468-15.2015. J Neurosci. 2015. PMID: 26490876 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

