Pleiotropic effects of miR-183~96~182 converge to regulate cell survival, proliferation and migration in medulloblastoma
- PMID: 22402744
- PMCID: PMC6172007
- DOI: 10.1007/s00401-012-0969-5
Pleiotropic effects of miR-183~96~182 converge to regulate cell survival, proliferation and migration in medulloblastoma
Abstract
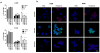
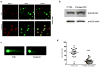
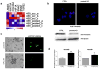
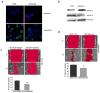
Medulloblastomas are the most common malignant brain tumors in children. Several large-scale genomic studies have detailed their heterogeneity, defining multiple subtypes with unique molecular profiles and clinical behavior. Increased expression of the miR-183~96~182 cluster of microRNAs has been noted in several subgroups, including the most clinically aggressive subgroup associated with genetic amplification of MYC. To understand the contribution of miR-183~96~182 to the pathogenesis of this aggressive subtype of medulloblastoma, we analyzed global gene expression and proteomic changes that occur upon modulation of miRNAs in this cluster individually and as a group in MYC-amplified medulloblastoma cells. Knockdown of the full miR-183~96~182 cluster results in enrichment of genes associated with apoptosis and dysregulation of the PI3K/AKT/mTOR signaling axis. Conversely, there is a relative enrichment of pathways associated with migration, metastasis and epithelial to mesenchymal transition, as well as pathways associated with dysfunction of DNA repair in cells with preserved miR-183 cluster expression. Immunocytochemistry and FACS analysis confirm induction of apoptosis upon knockdown of the miR-183 cluster. Importantly, cell-based migration and invasion assays verify the positive regulation of cell motility/migration by the miR-183 cluster, which is largely mediated by miR-182. We show that the effects on cell migration induced by the miR-183 cluster are coupled to the PI3K/AKT/mTOR pathway through differential regulation of AKT1 and AKT2 isoforms. Furthermore, we show that rapamycin inhibits cell motility/migration in medulloblastoma cells and phenocopies miR-183 cluster knockdown. Thus, the miR-183 cluster regulates multiple biological programs that converge to support the maintenance and metastatic potential of medulloblastoma.
Conflict of interest statement
Figures


References
-
- Abraham D, Jackson N, Gundara JS, Zhao J, Gill AJ, Delbridge L, Robinson BG, Sidhu SB. MicroRNA Profiling of Sporadic and Hereditary Medullary Thyroid Cancer Identifies Predictors of Nodal Metastasis, Prognosis. and Potential Therapeutic Targets Clin Cancer Res. 17(14):4772–4781. - PubMed
-
- Agiostratidou G, Hulit J, Phillips GR, Hazan RB. Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia. 2007;12(2-3):127–133. - PubMed
-
- Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, Frohling S, Chan EM, Sos ML, Michel K, Mermel C, Silver SJ, Weir BA, Reiling JH, Sheng Q, Gupta PB, Wadlow RC, Le H, Hoersch S, Wittner BS, Ramaswamy S, Livingston DM, Sabatini DM, Meyerson M, Thomas RK, Lander ES, Mesirov JP, Root DE, Gilliland DG, Jacks T, Hahn WC. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462(7269):108–112. - PMC - PubMed
-
- Bunt J, de Haas TG, Hasselt NE, Zwijnenburg DA, Koster J, Versteeg R, Kool M. Regulation of cell cycle genes and induction of senescence by overexpression of OTX2 in medulloblastoma cell lines. Mol Cancer Res. 8(10):1344–1357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

