Antiparallel β-sheet architecture in Iowa-mutant β-amyloid fibrils
- PMID: 22403062
- PMCID: PMC3311365
- DOI: 10.1073/pnas.1111305109
Antiparallel β-sheet architecture in Iowa-mutant β-amyloid fibrils
Abstract
Wild-type, full-length (40- and 42-residue) amyloid β-peptide (Aβ) fibrils have been shown by a variety of magnetic resonance techniques to contain cross-β structures in which the β-sheets have an in-register parallel supramolecular organization. In contrast, recent studies of fibrils formed in vitro by the Asp23-to-Asn mutant of 40-residue Aβ (D23N-Aβ(1-40)), which is associated with early onset neurodegeneration, indicate that D23N-Aβ(1-40) fibrils can contain either parallel or antiparallel β-sheets. We report a protocol for producing structurally pure antiparallel D23N-Aβ(1-40) fibril samples and a series of solid state nuclear magnetic resonance and electron microscopy measurements that lead to a specific model for the antiparallel D23N-Aβ(1-40) fibril structure. This model reveals how both parallel and antiparallel cross-β structures can be constructed from similar peptide monomer conformations and stabilized by similar sets of interactions, primarily hydrophobic in nature. We find that antiparallel D23N-Aβ(1-40) fibrils are thermodynamically metastable with respect to conversion to parallel structures, propagate less efficiently than parallel fibrils in seeded fibril growth, and therefore must nucleate more efficiently than parallel fibrils in order to be observable. Experiments in neuronal cell cultures indicate that both antiparallel and parallel D23N-Aβ(1-40) fibrils are cytotoxic. Thus, our antiparallel D23N-Aβ(1-40) fibril model represents a specific "toxic intermediate" in the aggregation process of a disease-associated Aβ mutant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
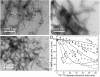
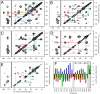

 chemical shifts of I31 and V18 (dashed lines). Strong I31 Cα/M35 Cα and V18 Cα/F20 Cα crosspeaks indicate antiparallel β-sheets. (C) 2D RAD spectrum of SFg2 fibril sample C with 1D slices at the I32
chemical shifts of I31 and V18 (dashed lines). Strong I31 Cα/M35 Cα and V18 Cα/F20 Cα crosspeaks indicate antiparallel β-sheets. (C) 2D RAD spectrum of SFg2 fibril sample C with 1D slices at the I32  , L34
, L34  , and A21
, and A21  chemical shifts (dashed lines). Arrows indicate interresidue crosspeaks.
chemical shifts (dashed lines). Arrows indicate interresidue crosspeaks.
References
-
- Kheterpal I, Williams A, Murphy C, Bledsoe B, Wetzel R. Structural features of the Aβ amyloid fibril elucidated by limited proteolysis. Biochemistry. 2001;40:11757–11767. - PubMed
-
- Chimon S, et al. Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer’s β-amyloid. Nat Struct Mol Biol. 2007;14:1157–1164. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

