Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis
- PMID: 22403066
- PMCID: PMC3323962
- DOI: 10.1073/pnas.1114931109
Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis
Abstract
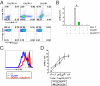
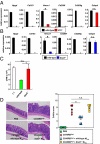
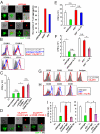
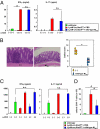
Adequate activation of CD4(+) T lymphocytes is essential for host defense against invading pathogens; however, exaggerated activity of effector CD4(+) T cells induces tissue damage, leading to inflammatory disorders such as inflammatory bowel diseases. Several unique subsets of intestinal innate immune cells have been identified. However, the direct involvement of innate immune cell subsets in the suppression of T-cell-dependent intestinal inflammation is poorly understood. Here, we report that intestinal CX(3)C chemokine receptor 1(high) (CX(3)CR1(high)) CD11b(+) CD11c(+) cells are responsible for prevention of intestinal inflammation through inhibition of T-cell responses. These cells inhibit CD4(+) T-cell proliferation in a cell contact-dependent manner and prevent T-cell-dependent colitis. The suppressive activity is abrogated in the absence of the IL-10/Stat3 pathway. These cells inhibit T-cell proliferation by two steps. Initially, CX(3)CR1(high) CD11b(+) CD11c(+) cells preferentially interact with T cells through highly expressed intercellular adhesion molecule-1/vascular cell adhesion molecule-1; then, they fail to activate T cells because of defective expression of CD80/CD86. The IL-10/Stat3 pathway mediates the reduction of CD80/CD86 expression. Transfer of wild-type CX(3)CR1(high) CD11b(+) CD11c(+) cells prevents development of colitis in myeloid-specific Stat3-deficient mice. Thus, these cells are regulatory myeloid cells that are responsible for maintaining intestinal homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. - PubMed
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. - PubMed
-
- Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: How do they suppress immune responses? Int Immunol. 2009;21:1105–1111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

