Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism
- PMID: 22403346
- PMCID: PMC3365407
- DOI: 10.1152/japplphysiol.00060.2012
Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism
Abstract
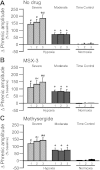
Acute intermittent hypoxia [AIH; 3, 5-min episodes; 35-45 mmHg arterial PO(2) (Pa(O(2)))] elicits serotonin-dependent phrenic long-term facilitation (pLTF), a form of phrenic motor facilitation (pMF) initiated by G(q) protein-coupled metabotropic 5-HT(2) receptors. An alternate pathway to pMF is induced by G(s) protein-coupled metabotropic receptors, including adenosine A(2A) receptors. AIH-induced pLTF is dominated by the serotonin-dependent pathway and is actually restrained via inhibition from the adenosine-dependent pathway. Here, we hypothesized that severe AIH shifts pLTF from a serotonin-dependent to an adenosine-dependent form of pMF. pLTF induced by severe (25-30 mmHg Pa(O(2))) and moderate (45-55 mmHg Pa(O(2))) AIH were compared in anesthetized rats, with and without intrathecal (C4) spinal A(2A) (MSX-3, 130 ng/kg, 12 μl) or 5-HT receptor antagonist (methysergide, 300 μg/kg, 15 μl) injections. During severe, but not moderate AIH, progressive augmentation of the phrenic response during hypoxic episodes was observed. Severe AIH (78% ± 8% 90 min post-AIH, n = 6) elicited greater pLTF vs. moderate AIH (41% ± 12%, n = 8; P < 0.05). MSX-3 (28% ± 6%; n = 6; P < 0.05) attenuated pLTF following severe AIH, but enhanced pLTF following moderate AIH (86% ± 26%; n = 8; P < 0.05). Methysergide abolished pLTF after moderate AIH (12% ± 5%; n = 6; P = 0.035), but had no effect after severe AIH (66 ± 13%; n = 5; P > 0.05). Thus severe AIH shifts pLTF from a serotonin-dependent to an adenosine-dependent mechanism; the adenosinergic pathway inhibits the serotonergic pathway following moderate AIH. Here we demonstrate a novel adenosine-dependent pathway to pLTF following severe AIH. Shifts in the mechanisms of respiratory plasticity provide the ventilatory control system greater flexibility as challenges that differ in severity are confronted.
Figures





Similar articles
-
Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation.Neuroscience. 2013 Oct 10;250:632-43. doi: 10.1016/j.neuroscience.2013.06.068. Epub 2013 Jul 11. Neuroscience. 2013. PMID: 23850591 Free PMC article.
-
Sustained Hypoxia Elicits Competing Spinal Mechanisms of Phrenic Motor Facilitation.J Neurosci. 2016 Jul 27;36(30):7877-85. doi: 10.1523/JNEUROSCI.4122-15.2016. J Neurosci. 2016. PMID: 27466333 Free PMC article.
-
Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia.J Physiol. 2010 Jan 1;588(Pt 1):255-66. doi: 10.1113/jphysiol.2009.180075. Epub 2009 Nov 9. J Physiol. 2010. PMID: 19900961 Free PMC article.
-
Circulatory control of phrenic motor plasticity.Respir Physiol Neurobiol. 2019 Jul;265:19-23. doi: 10.1016/j.resp.2019.01.004. Epub 2019 Jan 11. Respir Physiol Neurobiol. 2019. PMID: 30639504 Free PMC article. Review.
-
Hypoxia-induced phrenic long-term facilitation: emergent properties.Ann N Y Acad Sci. 2013 Mar;1279:143-53. doi: 10.1111/nyas.12085. Ann N Y Acad Sci. 2013. PMID: 23531012 Free PMC article. Review.
Cited by
-
Differential mechanisms are required for phrenic long-term facilitation over the course of motor neuron loss following CTB-SAP intrapleural injections.Exp Neurol. 2020 Dec;334:113460. doi: 10.1016/j.expneurol.2020.113460. Epub 2020 Sep 9. Exp Neurol. 2020. PMID: 32916172 Free PMC article.
-
Synergy between Acute Intermittent Hypoxia and Task-Specific Training.Exerc Sport Sci Rev. 2020 Jul;48(3):125-132. doi: 10.1249/JES.0000000000000222. Exerc Sport Sci Rev. 2020. PMID: 32412926 Free PMC article. Review.
-
The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation.J Appl Physiol (1985). 2013 Jan 1;114(1):52-65. doi: 10.1152/japplphysiol.00985.2012. Epub 2012 Nov 8. J Appl Physiol (1985). 2013. PMID: 23139361 Free PMC article.
-
Serotonin and Adenosine G-protein Coupled Receptor Signaling for Ventilatory Acclimatization to Sustained Hypoxia.Front Physiol. 2018 Jul 6;9:860. doi: 10.3389/fphys.2018.00860. eCollection 2018. Front Physiol. 2018. PMID: 30072908 Free PMC article.
-
Nonsteroidal anti-inflammatory drug (ketoprofen) delivery differentially impacts phrenic long-term facilitation in rats with motor neuron death induced by intrapleural CTB-SAP injections.Exp Neurol. 2022 Jan;347:113892. doi: 10.1016/j.expneurol.2021.113892. Epub 2021 Oct 9. Exp Neurol. 2022. PMID: 34634309 Free PMC article.
References
-
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 - PubMed
-
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

