Postnatal development and activation of L-type Ca2+ currents in locus ceruleus neurons: implications for a role for Ca2+ in central chemosensitivity
- PMID: 22403350
- PMCID: PMC3365409
- DOI: 10.1152/japplphysiol.01585.2011
Postnatal development and activation of L-type Ca2+ currents in locus ceruleus neurons: implications for a role for Ca2+ in central chemosensitivity
Abstract
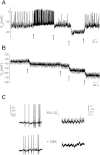
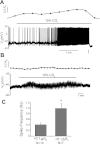
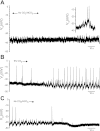
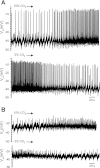
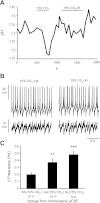
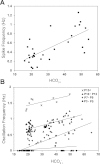
Little is known about the role of Ca(2+) in central chemosensitive signaling. We use electrophysiology to examine the chemosensitive responses of tetrodotoxin (TTX)-insensitive oscillations and spikes in neurons of the locus ceruleus (LC), a chemosensitive region involved in respiratory control. We show that both TTX-insensitive spikes and oscillations in LC neurons are sensitive to L-type Ca(2+) channel inhibition and are activated by increased CO(2)/H(+). Spikes appear to arise from L-type Ca(2+) channels on the soma whereas oscillations arise from L-type Ca(2+) channels that are distal to the soma. In HEPES-buffered solution (nominal absence of CO(2)/HCO(3)(-)), acidification does not activate either oscillations or spikes. When CO(2) is increased while extracellular pH is held constant by elevated HCO(3)(-), both oscillation and spike frequency increase. Furthermore, plots of both oscillation and spike frequency vs. intracellular [HCO(3)(-)]show a strong linear correlation. Increased frequency of TTX-insensitive spikes is associated with increases in intracellular Ca(2+) concentrations. Finally, both the appearance and frequency of TTX-insensitive spikes and oscillations increase over postnatal ages day 3-16. Our data suggest that 1) L-type Ca(2+) currents in LC neurons arise from channel populations that reside in different regions of the neuron, 2) these L-type Ca(2+) currents undergo significant postnatal development, and 3) the activity of these L-type Ca(2+) currents is activated by increased CO(2) through a HCO(3)(-)-dependent mechanism. Thus the activity of L-type Ca(2+) channels is likely to play a role in the chemosensitive response of LC neurons and may underlie significant changes in LC neuron chemosensitivity during neonatal development.
Figures




Similar articles
-
Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels.Am J Physiol Cell Physiol. 2003 Jan;284(1):C145-55. doi: 10.1152/ajpcell.00346.2002. Epub 2002 Sep 18. Am J Physiol Cell Physiol. 2003. PMID: 12388081
-
Central Nervous System-Toxic Lidocaine Concentrations Unmask L-Type Ca²⁺ Current-Mediated Action Potentials in Rat Thalamocortical Neurons: An In Vitro Mechanism of Action Study.Anesth Analg. 2016 May;122(5):1360-9. doi: 10.1213/ANE.0000000000001158. Anesth Analg. 2016. PMID: 26771269
-
A concerted action of L- and T-type Ca(2+) channels regulates locus coeruleus pacemaking.Mol Cell Neurosci. 2015 Sep;68:293-302. doi: 10.1016/j.mcn.2015.08.012. Epub 2015 Aug 25. Mol Cell Neurosci. 2015. PMID: 26319746
-
The locus coeruleus and central chemosensitivity.Respir Physiol Neurobiol. 2010 Oct 31;173(3):264-73. doi: 10.1016/j.resp.2010.04.024. Epub 2010 May 8. Respir Physiol Neurobiol. 2010. PMID: 20435170 Free PMC article. Review.
-
Exploring the dominant role of Cav1 channels in signalling to the nucleus.Biosci Rep. 2012 Dec 20;33(1):97-101. doi: 10.1042/BSR20120099. Biosci Rep. 2012. PMID: 23088728 Free PMC article. Review.
Cited by
-
Criteria for central respiratory chemoreceptors: experimental evidence supporting current candidate cell groups.Front Physiol. 2023 Sep 1;14:1241662. doi: 10.3389/fphys.2023.1241662. eCollection 2023. Front Physiol. 2023. PMID: 37719465 Free PMC article. Review.
-
Calcium, Bioenergetics, and Parkinson's Disease.Cells. 2020 Sep 8;9(9):2045. doi: 10.3390/cells9092045. Cells. 2020. PMID: 32911641 Free PMC article. Review.
-
Environmentally induced return to juvenile-like chemosensitivity in the respiratory control system of adult bullfrog, Lithobates catesbeianus.J Physiol. 2016 Nov 1;594(21):6349-6367. doi: 10.1113/JP272777. Epub 2016 Sep 15. J Physiol. 2016. PMID: 27444338 Free PMC article.
-
Locus Coeruleus Neurons' Firing Pattern Is Regulated by ERG Voltage-Gated K+ Channels.Int J Mol Sci. 2022 Dec 5;23(23):15334. doi: 10.3390/ijms232315334. Int J Mol Sci. 2022. PMID: 36499661 Free PMC article.
-
TRPM4 Contributes to Subthreshold Membrane Potential Oscillations in Multiple Mouse Pacemaker Neurons.eNeuro. 2021 Nov 17;8(6):ENEURO.0212-21.2021. doi: 10.1523/ENEURO.0212-21.2021. Print 2021 Nov-Dec. eNeuro. 2021. PMID: 34732535 Free PMC article.
References
-
- Aghajanian GK, Vandermaelen CP, Andrade R. Intracellular studies on the role of calcium in regulating the activity and reactivity of locus coeruleus neurons in vivo. Brain Res 273: 237–243, 1983 - PubMed
-
- Andrzejewski M, Mückenhoff K, Scheid P, Ballantyne D. Synchronized rhythms in chemosensitive neurons of the locus coeruleus in the absence of chemical synaptic transmission. Respir Physiol 129: 123–140, 2001 - PubMed
-
- Ballantyne D, Andrzejewski M, Muckenhoff K, Scheid P. Rhythms, synchrony and electrical coupling in the locus coeruleus. Respir Physiol Neurobiol 143: 199–214, 2004 - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000 - PubMed
-
- Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflügers Arch 455: 1119–1128, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

