The role of optical coherence tomography in coronary intervention
- PMID: 22403493
- PMCID: PMC3295975
- DOI: 10.3904/kjim.2012.27.1.1
The role of optical coherence tomography in coronary intervention
Abstract
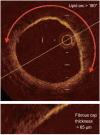
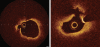
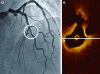
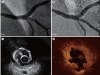
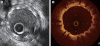
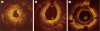
Optical coherence tomography (OCT) is an optical analog of intravascular ultrasound (IVUS) that can be used to examine the coronary arteries and has 10-fold higher resolution than IVUS. Based on polarization properties, OCT can differentiate tissue characteristics (fibrous, calcified, or lipid-rich plaque) and identify thin-cap fibroatheroma. Because of the strong attenuation of light by blood, OCT systems required the removal of blood during OCT examinations. A recently developed frequency-domain OCT system has a faster frame rate and pullback speed, making the OCT procedure more user-friendly and not requiring proximal balloon occlusion. During percutaneous coronary intervention (PCI), OCT can provide detailed information (dissection, tissue prolapse, thrombi, and incomplete stent apposition [ISA]). At follow-up examinations after stent implantation, stent strut coverage and ISA can be assessed. Several OCT studies have demonstrated delayed neointimal coverage following drug-eluting stent (DES) implantation vs. bare metal stent (BMS) placement. While newer DESs promote more favorable vascular healing, the clinical implications remain unknown. Recent OCT studies have provided insights into restenotic tissue characteristics; DES restenotic morphologies differ from those with BMSs. OCT is a novel, promising imaging modality; with more in-depth assessments of its use, it may impact clinical outcomes in patients with symptomatic coronary artery disease.
Keywords: Angioplasty; Coronary disease; Stents; Tomography, optical coherence; Ultrasonography, interventional.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
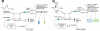



References
-
- Yamaguchi T, Terashima M, Akasaka T, et al. Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am J Cardiol. 2008;101:562–567. - PubMed
-
- Kataiwa H, Tanaka A, Kitabata H, et al. Head to head comparison between the conventional balloon occlusion method and the non-occlusion method for optical coherence tomography. Int J Cardiol. 2011;146:186–190. - PubMed
-
- Prati F, Cera M, Ramazzotti V, et al. From bench to bedside: a novel technique of acquiring OCT images. Circ J. 2008;72:839–843. - PubMed
-
- Barlis P, Schmitt JM. Current and future developments in intracoronary optical coherence tomography imaging. EuroIntervention. 2009;4:529–533. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
