Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis
- PMID: 22403663
- PMCID: PMC3293815
- DOI: 10.1371/journal.pone.0032482
Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis
Abstract
Background: Despite the widespread use of interferon-γ release assays (IGRAs), their role in diagnosing tuberculosis and targeting preventive therapy in HIV-infected patients remains unclear. We conducted a comprehensive systematic review to contribute to the evidence-based practice in HIV-infected people.
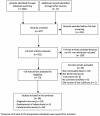
Methodology/principal findings: We searched MEDLINE, Cochrane, and Biomedicine databases to identify articles published between January 2005 and July 2011 that assessed QuantiFERON®-TB Gold In-Tube (QFT-GIT) and T-SPOT®.TB (T-SPOT.TB) in HIV-infected adults. We assessed their accuracy for the diagnosis of tuberculosis and incident active tuberculosis, and the proportion of indeterminate results. The search identified 38 evaluable studies covering a total of 6514 HIV-infected participants. The pooled sensitivity and specificity for tuberculosis were 61% and 72% for QFT-GIT, and 65% and 70% for T-SPOT.TB. The cumulative incidence of subsequent active tuberculosis was 8.3% for QFT-GIT and 10% for T-SPOT.TB in patients tested positive (one study each), and 0% for QFT-GIT (two studies) and T-SPOT.TB (one study) respectively in those tested negative. Pooled indeterminate rates were 8.2% for QFT-GIT and 5.9% for T-SPOT.TB. Rates were higher in high burden settings (12.0% for QFT-GIT and 7.7% for T-SPOT.TB) than in low-intermediate burden settings (3.9% for QFT-GIT and 4.3% for T-SPOT.TB). They were also higher in patients with CD4(+) T-cell count <200 (11.6% for QFT-GIT and 11.4% for T-SPOT.TB) than in those with CD4(+) T-cell count ≥ 200 (3.1% for QFT-GIT and 7.9% for T-SPOT.TB).
Conclusions/significance: IGRAs have suboptimal accuracy for confirming or ruling out active tuberculosis disease in HIV-infected adults. While their predictive value for incident active tuberculosis is modest, a negative QFT-GIT implies a very low short- to medium-term risk. Identifying the factors associated with indeterminate results will help to optimize the use of IGRAs in clinical practice, particularly in resource-limited countries with a high prevalence of HIV-coinfection.
Conflict of interest statement
Figures





References
-
- Giraldi E, Antonucci G, Vanacore P, Palmieri F, Matteelli A, et al. Tuberculosis in HIV-infected persons in the context of wide availability of highly active antiretroviral therapy. Eur Respir J. 2004;24:11–17. - PubMed
-
- World Health Organization website. Available: http://whqlibdoc.who.int/publications/2011/9789241564380_eng.pdf. Accessed 2011 September 22.
-
- Centers for Disease Control and Prevention. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR Early Release. 2009;58:1–207.
-
- Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, et al. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7. - PubMed
-
- Markowitz N, Hansen NI, Wilcosky TC, Hopewell PC, Glassroth J, et al. Tuberculin and anergy testing in HIV seropositive and HIV seronegative persons. Pulmonary complications of HIV infection study group. Ann Intern Med. 1993;119:185–193. - PubMed

