Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer's disease
- PMID: 22403710
- PMCID: PMC3293895
- DOI: 10.1371/journal.pone.0032792
Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer's disease
Abstract
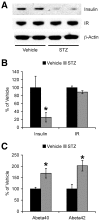
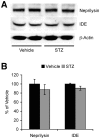
Although evidence is accumulating that diabetes mellitus is an important risk factor for sporadic Alzheimer's disease (AD), the mechanisms by which defects in insulin signaling may lead to the acceleration of AD progression remain unclear. In this study, we applied streptozotocin (STZ) to induce experimental diabetes in AD transgenic mice (5XFAD model) and investigated how insulin deficiency affects the β-amyloidogenic processing of amyloid precursor protein (APP). Two and half months after 5XFAD mice were treated with STZ (90 mg/kg, i.p., once daily for two consecutive days), they showed significant reductions in brain insulin levels without changes in insulin receptor expression. Concentrations of cerebral amyloid-β peptides (Aβ40 and Aβ42) were significantly increased in STZ-treated 5XFAD mice as compared with vehicle-treated 5XFAD controls. Importantly, STZ-induced insulin deficiency upregulated levels of both β-site APP cleaving enzyme 1 (BACE1) and full-length APP in 5XFAD mouse brains, which was accompanied by dramatic elevations in the β-cleaved C-terminal fragment (C99). Interestingly, BACE1 mRNA levels were not affected, whereas phosphorylation of the translation initiation factor eIF2α, a mechanism proposed to mediate the post-transcriptional upregulation of BACE1, was significantly elevated in STZ-treated 5XFAD mice. Meanwhile, levels of GGA3, an adapter protein responsible for sorting BACE1 to lysosomal degradation, are indistinguishable between STZ- and vehicle-treated 5XFAD mice. Moreover, STZ treatments did not affect levels of Aβ-degrading enzymes such as neprilysin and insulin-degrading enzyme (IDE) in 5XFAD brains. Taken together, our findings provide a mechanistic foundation for a link between diabetes and AD by demonstrating that insulin deficiency may change APP processing to favor β-amyloidogenesis via the translational upregulation of BACE1 in combination with elevations in its substrate, APP.
Conflict of interest statement
Figures


Similar articles
-
Sex- and brain region-specific acceleration of β-amyloidogenesis following behavioral stress in a mouse model of Alzheimer's disease.Mol Brain. 2010 Nov 8;3:34. doi: 10.1186/1756-6606-3-34. Mol Brain. 2010. PMID: 21059265 Free PMC article.
-
A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice.Mol Brain. 2015 Mar 25;8:19. doi: 10.1186/s13041-015-0110-5. Mol Brain. 2015. PMID: 25884928 Free PMC article.
-
BACE1 elevation engendered by GGA3 deletion increases β-amyloid pathology in association with APP elevation and decreased CHL1 processing in 5XFAD mice.Mol Neurodegener. 2018 Feb 2;13(1):6. doi: 10.1186/s13024-018-0239-7. Mol Neurodegener. 2018. PMID: 29391027 Free PMC article.
-
The β-Secretase Enzyme BACE1: A Biochemical Enigma for Alzheimer's Disease.CNS Neurol Disord Drug Targets. 2020;19(3):184-194. doi: 10.2174/1871527319666200526144141. CNS Neurol Disord Drug Targets. 2020. PMID: 32452328 Review.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
Cited by
-
Crosstalk between neurological, cardiovascular, and lifestyle disorders: insulin and lipoproteins in the lead role.Pharmacol Rep. 2022 Oct;74(5):790-817. doi: 10.1007/s43440-022-00417-5. Epub 2022 Sep 23. Pharmacol Rep. 2022. PMID: 36149598 Review.
-
Insulin Resistance and Diabetes Mellitus in Alzheimer's Disease.Cells. 2021 May 18;10(5):1236. doi: 10.3390/cells10051236. Cells. 2021. PMID: 34069890 Free PMC article. Review.
-
The Promising Potency of Sodium-Glucose Cotransporter 2 Inhibitors in the Prevention of and as Treatment for Cognitive Impairment Among Type 2 Diabetes Patients.Biomedicines. 2024 Dec 6;12(12):2783. doi: 10.3390/biomedicines12122783. Biomedicines. 2024. PMID: 39767690 Free PMC article. Review.
-
GLP-1 Analogs, SGLT-2, and DPP-4 Inhibitors: A Triad of Hope for Alzheimer's Disease Therapy.Biomedicines. 2023 Nov 12;11(11):3035. doi: 10.3390/biomedicines11113035. Biomedicines. 2023. PMID: 38002034 Free PMC article. Review.
-
Alterations in the polysialylated neural cell adhesion molecule and retinal ganglion cell density in mice with diabetic retinopathy.Int J Ophthalmol. 2018 Oct 18;11(10):1608-1615. doi: 10.18240/ijo.2018.10.06. eCollection 2018. Int J Ophthalmol. 2018. PMID: 30364237 Free PMC article.
References
-
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Holscher C. Diabetes as a risk factor for Alzheimer's disease: insulin signalling impairment in the brain as an alternative model of Alzheimer's disease. Biochem Soc Trans. 2011;39:891–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

