Receptor signaling clusters in the immune synapse
- PMID: 22404679
- PMCID: PMC4000727
- DOI: 10.1146/annurev-biophys-042910-155238
Receptor signaling clusters in the immune synapse
Abstract
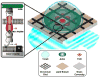
Signaling processes between various immune cells involve large-scale spatial reorganization of receptors and signaling molecules within the cell-cell junction. These structures, now collectively referred to as immune synapses, interleave physical and mechanical processes with the cascades of chemical reactions that constitute signal transduction systems. Molecular level clustering, spatial exclusion, and long-range directed transport are all emerging as key regulatory mechanisms. The study of these processes is drawing researchers from physical sciences to join the effort and represents a rapidly growing branch of biophysical chemistry. Recent advances in physical and quantitative analyses of signaling within the immune synapses are reviewed here.
Figures





References
-
- Al-Alwan MM, Liwski RS, Haeryfar SMM, Baldridge WH, Hoskin DW, et al. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J Immunol. 2003;171:4479–83. - PubMed
-
- Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–56. - PubMed
-
- Allen PG, Dawidowicz EA. Phagocytosis in Acanthamoeba: I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J Cell Physiol. 1990;145:508–13. - PubMed
-
- Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol. 2000;1:156–62. - PubMed
-
- Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

