Specific tumor-stroma interactions of EBV-positive Burkitt's lymphoma cells in the chick chorioallantoic membrane
- PMID: 22404859
- PMCID: PMC3325879
- DOI: 10.1186/2045-824X-4-3
Specific tumor-stroma interactions of EBV-positive Burkitt's lymphoma cells in the chick chorioallantoic membrane
Abstract
Background: Burkitt's lymphoma (BL) is an aggressive Non-Hodgkin lymphoma. Epstein-Barr Virus (EBV) is able to transform B cells and is a causative infectious agent in BL. The precise role of EBV in lymphoma progression is still unclear. Most investigations have concentrated on cell autonomous functions of EBV in B cells. Functions of the local environment in BL progression have rarely been studied, mainly due to the lack of appropriate in vivo models. Therefore, we inoculated different human BL cell-lines onto the chorioallantoic membrane (CAM) of embryonic day 10 (ED10) chick embryos and re-incubated until ED14 and ED17.
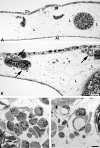
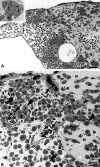
Results: All cell-lines formed solid tumors. However, we observed strong differences in the behavior of EBV+ and EBV- cell-lines. Tumor borders of EBV+ cells were very fuzzy and numerous cells migrated into the CAM. In EBV- tumors, the borders were much better defined. In contrast to EBV- cells, the EBV+ cells induced massive immigration of chick leukocytes at the tumor borders and the development of granulation tissue with large numbers of blood vessels and lymphatics, although the expression of pro- and anti-angiogenic forms of Vascular Endothelial Growth Factors/receptors was the same in all BL cell-lines tested. The EBV+ cell-lines massively disseminated via the lymphatics and completely occluded them.
Conclusions: Our data suggest that the EBV+ cells attract pro-angiogenic leukocytes, which then induce secondary tumor-stroma interactions contributing to the progression of BL. We show that the CAM is a highly suitable in vivo model to study the differential behavior of BL cell-lines.
Figures








References
-
- Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N. et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. - DOI - PubMed
-
- Kaiser U, Uebelacker I, Abel U, Birkmann J, Trumper L, Schmalenberg H, Karakas T, Metzner B, Hossfeld DK, Bischoff HG. et al. Randomized study to evaluate the use of high-dose therapy as part of primary treatment for "aggressive" lymphoma. J Clin Oncol. 2002;20:4413–4419. doi: 10.1200/JCO.2002.07.075. - DOI - PubMed
-
- Wunderlich A, Kloess M, Reiser M, Rudolph C, Truemper L, Bittner S, Schmalenberg H, Schmits R, Pfreundschuh M, Loeffler M. Practicability and acute haematological toxicity of 2- and 3-weekly CHOP and CHOEP chemotherapy for aggressive non-Hodgkin's lymphoma: results from the NHL-B trial of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL) Ann Oncol. 2003;14:881–893. doi: 10.1093/annonc/mdg249. - DOI - PubMed
-
- Chene A, Donati D, Orem J, Mbidde ER, Kironde F, Wahlgren M, Bejarano MT. Endemic Burkitt's lymphoma as a polymicrobial disease: new insights on the interaction between Plasmodium falciparum and Epstein-Barr virus. Semin Cancer Biol. 2009;19:411–420. doi: 10.1016/j.semcancer.2009.10.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources

