Glucocorticoids suppress selected components of the senescence-associated secretory phenotype
- PMID: 22404905
- PMCID: PMC3387333
- DOI: 10.1111/j.1474-9726.2012.00818.x
Glucocorticoids suppress selected components of the senescence-associated secretory phenotype
Abstract
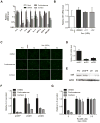
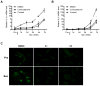
Cellular senescence suppresses cancer by arresting the proliferation of cells at risk for malignant transformation. Recently, senescent cells were shown to secrete numerous cytokines, growth factors, and proteases that can alter the tissue microenvironment and may promote age-related pathology. To identify small molecules that suppress the senescence-associated secretory phenotype (SASP), we developed a screening protocol using normal human fibroblasts and a library of compounds that are approved for human use. Among the promising library constituents was the glucocorticoid corticosterone. Both corticosterone and the related glucocorticoid cortisol decreased the production and secretion of selected SASP components, including several pro-inflammatory cytokines. Importantly, the glucocorticoids suppressed the SASP without reverting the tumor suppressive growth arrest and were efficacious whether cells were induced to senesce by ionizing radiation or strong mitogenic signals delivered by oncogenic RAS or MAP kinase kinase 6 overexpression. Suppression of the prototypical SASP component IL-6 required the glucocorticoid receptor, which, in the presence of ligand, inhibited IL-1α signaling and NF-κB transactivation activity. Accordingly, co-treatments combining glucocorticoids with the glucocorticoid antagonist RU-486 or recombinant IL-1α efficiently reestablished NF-κB transcriptional activity and IL-6 secretion. Our findings demonstrate feasibility of screening for compounds that inhibit the effects of senescent cells. They further show that glucocorticoids inhibit selected components of the SASP and suggest that corticosterone and cortisol, two FDA-approved drugs, might exert their effects in part by suppressing senescence-associated inflammation.
© 2012 The Authors. Aging Cell © 2012 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Conflict of interest statement
The authors report no financial or other conflict of interest relevant to the subject of this article.
Figures


References
-
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Furnagalli M, DaCosta M, Brown C, Popov N, Takastu, Yabuta N, Melamed J, d’Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- Adams PD. Healing and hurting: molecular mechanisms, functions and pathologies of cellular senescence. Molec Cell. 2009;36:2–14. - PubMed
-
- Beausejour CM, Campisi J. Ageing: balancing regeneration and cancer. Nature. 2006;443:404–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

