Actin network growth under load
- PMID: 22404927
- PMCID: PMC3296029
- DOI: 10.1016/j.bpj.2012.01.030
Actin network growth under load
Abstract
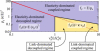
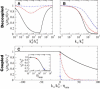
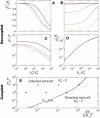
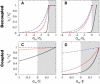
Many processes in eukaryotic cells, including the crawling motion of the whole cell, rely on the growth of branched actin networks from surfaces. In addition to their well-known role in generating propulsive forces, actin networks can also sustain substantial pulling loads thanks to their persistent attachment to the surface from which they grow. The simultaneous network elongation and surface attachment inevitably generate a force that opposes network growth. Here, we study the local dynamics of a growing actin network, accounting for simultaneous network elongation and surface attachment, and show that there exist several dynamical regimes that depend on both network elasticity and the kinetic parameters of actin polymerization. We characterize this in terms of a phase diagram and provide a connection between mesoscopic theories and the microscopic dynamics of an actin network at a surface. Our framework predicts the onset of instabilities that lead to the local detachment of the network and translate to oscillatory behavior and waves, as observed in many cellular phenomena and in vitro systems involving actin network growth, such as the saltatory dynamics of actin-propelled oil drops.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Bray D. Garland; New York: 1992. Cell Movements.
-
- Alberts B., Bray D., Watson J.D. 3rd ed. Garland; New York: 2004. Molecular Biology of the Cell.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

