Protein kinase CK2 localizes to sites of DNA double-strand break regulating the cellular response to DNA damage
- PMID: 22404984
- PMCID: PMC3316135
- DOI: 10.1186/1471-2199-13-7
Protein kinase CK2 localizes to sites of DNA double-strand break regulating the cellular response to DNA damage
Abstract
Background: The DNA-dependent protein kinase (DNA-PK) is a nuclear complex composed of a large catalytic subunit (DNA-PKcs) and a heterodimeric DNA-targeting subunit Ku. DNA-PK is a major component of the non-homologous end-joining (NHEJ) repair mechanism, which is activated in the presence of DNA double-strand breaks induced by ionizing radiation, reactive oxygen species and radiomimetic drugs. We have recently reported that down-regulation of protein kinase CK2 by siRNA interference results in enhanced cell death specifically in DNA-PKcs-proficient human glioblastoma cells, and this event is accompanied by decreased autophosphorylation of DNA-PKcs at S2056 and delayed repair of DNA double-strand breaks.
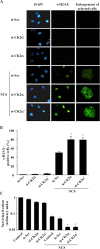
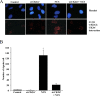
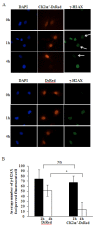
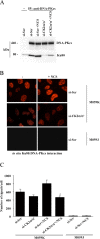
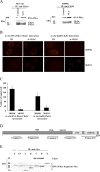
Results: In the present study, we show that CK2 co-localizes with phosphorylated histone H2AX to sites of DNA damage and while CK2 gene knockdown is associated with delayed DNA damage repair, its overexpression accelerates this process. We report for the first time evidence that lack of CK2 destabilizes the interaction of DNA-PKcs with DNA and with Ku80 at sites of genetic lesions. Furthermore, we show that CK2 regulates the phosphorylation levels of DNA-PKcs only in response to direct induction of DNA double-strand breaks.
Conclusions: Taken together, these results strongly indicate that CK2 plays a prominent role in NHEJ by facilitating and/or stabilizing the binding of DNA-PKcs and, possibly other repair proteins, to the DNA ends contributing to efficient DNA damage repair in mammalian cells.
Figures

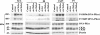
Similar articles
-
Regulation of DNA-dependent protein kinase by protein kinase CK2 in human glioblastoma cells.Oncogene. 2010 Nov 11;29(45):6016-26. doi: 10.1038/onc.2010.337. Epub 2010 Aug 16. Oncogene. 2010. PMID: 20711232
-
An Intrinsically Disordered APLF Links Ku, DNA-PKcs, and XRCC4-DNA Ligase IV in an Extended Flexible Non-homologous End Joining Complex.J Biol Chem. 2016 Dec 30;291(53):26987-27006. doi: 10.1074/jbc.M116.751867. Epub 2016 Nov 14. J Biol Chem. 2016. PMID: 27875301 Free PMC article.
-
Tyrosyl-DNA phosphodiesterase and the repair of 3'-phosphoglycolate-terminated DNA double-strand breaks.DNA Repair (Amst). 2009 Aug 6;8(8):901-11. doi: 10.1016/j.dnarep.2009.05.003. Epub 2009 Jun 7. DNA Repair (Amst). 2009. PMID: 19505854 Free PMC article.
-
A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation.DNA Repair (Amst). 2010 Dec 10;9(12):1307-14. doi: 10.1016/j.dnarep.2010.09.019. Epub 2010 Oct 28. DNA Repair (Amst). 2010. PMID: 21030321 Free PMC article. Review.
-
How to fix DNA breaks: new insights into the mechanism of non-homologous end joining.Biochem Soc Trans. 2023 Oct 31;51(5):1789-1800. doi: 10.1042/BST20220741. Biochem Soc Trans. 2023. PMID: 37787023 Free PMC article. Review.
Cited by
-
When PIP2 Meets p53: Nuclear Phosphoinositide Signaling in the DNA Damage Response.Front Cell Dev Biol. 2022 May 13;10:903994. doi: 10.3389/fcell.2022.903994. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646908 Free PMC article. Review.
-
Distinct roles of ATM and ATR in the regulation of ARP8 phosphorylation to prevent chromosome translocations.Elife. 2018 May 8;7:e32222. doi: 10.7554/eLife.32222. Elife. 2018. PMID: 29759113 Free PMC article.
-
Transcriptional activation of lipogenesis by insulin requires phosphorylation of MED17 by CK2.Sci Signal. 2017 Feb 21;10(467):eaai8596. doi: 10.1126/scisignal.aai8596. Sci Signal. 2017. PMID: 28223413 Free PMC article.
-
Ablation of beta subunit of protein kinase CK2 in mouse oocytes causes follicle atresia and premature ovarian failure.Cell Death Dis. 2018 May 1;9(5):508. doi: 10.1038/s41419-018-0505-1. Cell Death Dis. 2018. PMID: 29725001 Free PMC article.
-
Phosphorylation of xeroderma pigmentosum group C regulates ultraviolet-induced DNA damage repair.Nucleic Acids Res. 2018 Jun 1;46(10):5050-5060. doi: 10.1093/nar/gky239. Nucleic Acids Res. 2018. PMID: 29660033 Free PMC article.
References
-
- Helleday T, Petermann E, Lundin C, Hodgson B, Sharma R. DNA repair pathways as targets for cancer therapy. Nature Rev. 2008;8:193–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

