Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion
- PMID: 22405128
- PMCID: PMC4323149
- DOI: 10.3727/096368911X627381
Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion
Abstract
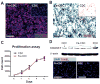
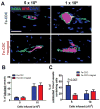
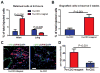
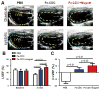
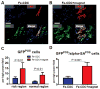
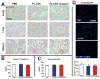
The efficiency of stem cell transplantation is limited by low cell retention. Intracoronary (IC) delivery is convenient and widely used but exhibits particularly low cell retention rates. We sought to improve IC cell retention by magnetic targeting. Rat cardiosphere-derived cells labeled with iron microspheres were injected into the left ventricular cavity of syngeneic rats during brief aortic clamping. Placement of a 1.3 Tesla magnet ~1 cm above the heart during and after cell injection enhanced cell retention at 24 h by 5.2-6.4-fold when 1, 3, or 5 × 10(5) cells were infused, without elevation of serum troponin I (sTnI) levels. Higher cell doses (1 or 2 × 10(6) cells) did raise sTnI levels, due to microvascular obstruction; in this range, magnetic enhancement did not improve cell retention. To assess efficacy, 5 × 10(5) iron-labeled, GFP-expressing cells were infused into rat hearts after 45 min ischemia/20 min reperfusion of the left anterior coronary artery, with and without a superimposed magnet. By quantitative PCR and optical imaging, magnetic targeting increased cardiac retention of transplanted cells at 24 h, and decreased migration into the lungs. The enhanced cell engraftment persisted for at least 3 weeks, at which time left ventricular remodeling was attenuated, and therapeutic benefit (ejection fraction) was higher, in the magnetic targeting group. Histology revealed more GFP(+) cardiomyocytes, Ki67(+) cardiomyocytes and GFP(-)/ckit(+) cells, and fewer TUNEL(+) cells, in hearts from the magnetic targeting group. In a rat model of ischemia/reperfusion injury, magnetically enhanced intracoronary cell delivery is safe and improves cell therapy outcomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Al Kindi A, Ge Y, Shum-Tim D, Chiu RC. Cellular cardiomyoplasty: Routes of cell delivery and retention. Front Biosci. 2008;13:2421–2434. - PubMed
-
- Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular mr imaging. Radiology. 2003;229:838–846. - PubMed
-
- Arbab AS, Jordan EK, Wilson LB, Yocum GT, Lewis BK, Frank JA. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum Gene Ther. 2004;15:351–360. - PubMed
-
- Assis AC, Carvalho JL, Jacoby BA, Ferreira RL, Castanheira P, Diniz SO, Cardoso VN, Goes AM, Ferreira AJ. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19:219–230. - PubMed
-
- Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clin Pharmacol Ther. 2009;85:548–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

