What does the concept of the stem cell niche really mean today?
- PMID: 22405133
- PMCID: PMC3298504
- DOI: 10.1186/1741-7007-10-19
What does the concept of the stem cell niche really mean today?
Figures



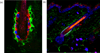


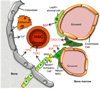

References
-
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

