FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development
- PMID: 22405201
- PMCID: PMC3326387
- DOI: 10.1016/j.neuron.2012.01.018
FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development
Abstract
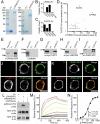
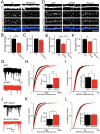
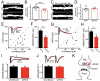
Latrophilins (LPHNs) are a small family of G protein-coupled receptors known to mediate the massive synaptic exocytosis caused by the black widow spider venom α-latrotoxin, but their endogenous ligands and function remain unclear. Mutations in LPHN3 are strongly associated with attention deficit hyperactivity disorder, suggesting a role for latrophilins in human cognitive function. Using affinity chromatography and mass spectrometry, we identify the FLRT family of leucine-rich repeat transmembrane proteins as endogenous postsynaptic ligands for latrophilins. We demonstrate that the FLRT3 and LPHN3 ectodomains interact with high affinity in trans and that interference with this interaction using soluble recombinant LPHN3, LPHN3 shRNA, or FLRT3 shRNA reduces excitatory synapse density in cultured neurons. In addition, reducing FLRT3 levels with shRNA in vivo decreases afferent input strength and dendritic spine number in dentate granule cells. These observations indicate that LPHN3 and its ligand FLRT3 play an important role in glutamatergic synapse development.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Allen Mouse Brain Atlas [Internet] Allen Institute for Brain Science. ©2009. Available from: http://mouse.brain-map.org.
-
- Arcos-Burgos M, Jain M, Acosta MT, Shively S, Stanescu H, Wallis D, Domene S, Velez JI, Karkera JD, Balog J, et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry. 2010;15:1053–1066. - PubMed
-
- Bottcher RT, Pollet N, Delius H, Niehrs C. The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nat Cell Biol. 2004;6:38–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

