Regulation of stress granules in virus systems
- PMID: 22405519
- PMCID: PMC3322245
- DOI: 10.1016/j.tim.2012.02.001
Regulation of stress granules in virus systems
Abstract
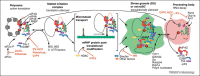
Virus infection initiates a number of cellular stress responses that modulate gene regulation and compartmentalization of RNA. Viruses must control host gene expression and the localization of viral RNAs to be successful parasites. RNA granules such as stress granules and processing bodies (PBs) contain translationally silenced messenger ribonucleoproteins (mRNPs) and serve as extensions of translation regulation in cells, storing transiently repressed mRNAs. New reports show a growing number of virus families modulate RNA granule function to maximize replication efficiency. This review summarizes recent advances in understanding the relationship between viruses and mRNA stress granules in animal cells and will discuss important questions that remain in this emerging field.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
References
-
- Lin W.J. Localization of AU-rich element-containing mRNA in cytoplasmic granules containing exosome subunits. J. Biol. Chem. 2007;282:19958–19968. - PubMed
-
- Krichevsky A.M., Kosik K.S. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

