An assessment of prime-boost vaccination schedules with AS03A -adjuvanted prepandemic H5N1 vaccines: a randomized study in European adults
- PMID: 22405557
- PMCID: PMC5780732
- DOI: 10.1111/j.1750-2659.2012.00349.x
An assessment of prime-boost vaccination schedules with AS03A -adjuvanted prepandemic H5N1 vaccines: a randomized study in European adults
Abstract
Background: Long-term persistence of immune response and safety of an H5N1 prepandemic influenza vaccine adjuvanted with AS03 (an α-tocopherol oil-in-water emulsion-based adjuvant system) was evaluated using various prime-boost schedules that mimicked potential pandemic scenarios (NCT00430521).
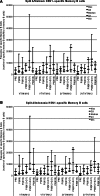
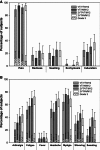
Methods: Five hundred and twelve healthy adults aged 18-60 years received primary vaccination with one or two doses (0, 21 days schedule) of the A/Vietnam/1194/2004 H5N1 vaccine followed by a booster dose (A/Vietnam/1194/2004 or A/Indonesia/05/2005 strain) six or twelve months later across eight randomized groups. Immunogenicity results by hemagglutination inhibition [HI] assay, microneutralization assay, and the cell-mediated immune response (CMI) are reported here for the four groups boosted at Month 12.
Results: A one-dose-adjuvanted primary administration followed 12 months later by a single-adjuvanted booster dose containing a heterologous vaccine strain met or exceeded all US and European criteria for both strains. Increasing the interval between the first and second dose (from 21 days to 12 months) resulted in stronger cross-reactive immune responses against the A/Indonesia/05/2005 strain. The HI antibody response against the two strains persisted for 6 months after the booster dose irrespective of the booster vaccine's strain. The neutralizing antibody responses and the CMI observed in the study population paralleled the HI immune response. Overall, the vaccine had a clinically acceptable safety profile.
Conclusion: The H5N1 vaccine in this study allowed for flexibility in the time interval between primary and booster vaccination and the use of a heterologous strain without impacting the strength of the humoral and cellular immune response to both vaccine strains.
© 2012 Blackwell Publishing Ltd.
Figures



References
-
- Melidou A, Gioula G, Exindari M, Chatzidimitriou D, Diza‐Mataftsi E. Influenza A (H5N1): an overview of the current situation. Eurosurveillance 2009; 145:220–226. - PubMed
-
- World Health Organization (WHO) . Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO, 6 May 2010. Global alert and response (GAR) Available at http://www.who.int/csr/disease/avian_influenza/country/cases_table_2011_... (Accessed 26 July 2011).
-
- World Health Organization (WHO) . Antigenic and genetic characteristics of influenza A(H5N1) and influenza A(H9N2) viruses for the development of candidate vaccine viruses for pandemic preparedness, February 2011. Available at http://www.who.int/csr/disease/avian_influenza/guidelines/2011_02_h5_h9_... (Accessed 23 May 2011). - PubMed
-
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus . Update on Avian Influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273. - PubMed
-
- European Committee for Proprietary Medicinal Products (CHMP) . Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006). European Agency for the Evaluation of Medicinal Products, January 24, 2007.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

