p53 and MDM2 are involved in the regulation of osteocalcin gene expression
- PMID: 22405968
- PMCID: PMC3319696
- DOI: 10.1016/j.yexcr.2012.02.022
p53 and MDM2 are involved in the regulation of osteocalcin gene expression
Erratum in
- Exp Cell Res. 2012 Oct 1;318(16):2153
Abstract
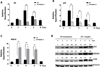
Osteocalcin (OC) is a major noncollagenous bone matrix protein and an osteoblast marker whose expression is limited to mature osteoblasts during the late differentiation stage. In previous studies we have shown osteosarcomas to lose p53 function with a corresponding loss of osteocalcin gene expression. Introduction of wild type p53 resulted in re expression of the osteocalcin gene. Using gel shift and chromatin immunoprecipitation assays, we have identified a putative p53 binding site within the rat OC promoter region and observed an increase in OC promoter activity when p53 accumulates using a CAT assay. The p53 inducible gene Mdm2 is a well-known downstream regulator of p53 levels. Our results showed a synergistic increase in the OC promoter activity when both p53 and MDM2 were transiently overexpressed. We further demonstrate that p53 is not degraded during overexpression of MDM2 protein. Increased OC expression was observed with concomitantly increased p53, VDR, and MDM2 levels in ROS17/2.8 cells during treatment with differentiation promoting (DP) media, but was significantly decreased when co-treated with DP media and the small molecule inhibitor of MDM2-p53 interaction, Nutlin-3. We have also observed a dramatic increase of the OC promoter activity in the presence of p53 and Mdm2 with inclusion of Cbfa-1 and p300 factors. Our results suggest that under some physiological conditions the oncoprotein MDM2 may cooperate with p53 to regulate the osteocalcin gene during osteoblastic differentiation.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Bone-specific transcription factor Runx2 interacts with the 1alpha,25-dihydroxyvitamin D3 receptor to up-regulate rat osteocalcin gene expression in osteoblastic cells.Mol Cell Biol. 2004 Oct;24(20):8847-61. doi: 10.1128/MCB.24.20.8847-8861.2004. Mol Cell Biol. 2004. PMID: 15456860 Free PMC article.
-
Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling.J Cell Biol. 2006 Mar 13;172(6):909-21. doi: 10.1083/jcb.200508130. J Cell Biol. 2006. PMID: 16533949 Free PMC article.
-
Pharmacological activation of the p53 pathway by nutlin-3 exerts anti-tumoral effects in medulloblastomas.Neuro Oncol. 2012 Jul;14(7):859-69. doi: 10.1093/neuonc/nos115. Epub 2012 May 16. Neuro Oncol. 2012. PMID: 22591662 Free PMC article.
-
Regulation of the bone-specific osteocalcin gene by p300 requires Runx2/Cbfa1 and the vitamin D3 receptor but not p300 intrinsic histone acetyltransferase activity.Mol Cell Biol. 2003 May;23(9):3339-51. doi: 10.1128/MCB.23.9.3339-3351.2003. Mol Cell Biol. 2003. PMID: 12697832 Free PMC article.
-
Pharmacologic activation of p53 by small-molecule MDM2 antagonists.Curr Pharm Des. 2011;17(6):560-8. doi: 10.2174/138161211795222603. Curr Pharm Des. 2011. PMID: 21391906 Free PMC article. Review.
Cited by
-
E3 ubiquitin ligases: key regulators of osteogenesis and potential therapeutic targets for bone disorders.Front Cell Dev Biol. 2024 Aug 15;12:1447093. doi: 10.3389/fcell.2024.1447093. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39211390 Free PMC article. Review.
-
Vitamin D directly regulates Mdm2 gene expression in osteoblasts.Biochem Biophys Res Commun. 2013 Jan 4;430(1):370-4. doi: 10.1016/j.bbrc.2012.11.003. Epub 2012 Nov 10. Biochem Biophys Res Commun. 2013. PMID: 23149414 Free PMC article.
-
Role of p53 deficiency in socket healing after tooth extractions.J Mol Histol. 2020 Feb;51(1):55-65. doi: 10.1007/s10735-020-09856-x. Epub 2020 Jan 31. J Mol Histol. 2020. PMID: 32006186
-
P53 regulation of osteoblast differentiation is mediated through specific microRNAs.Biochem Biophys Rep. 2021 Feb 1;25:100920. doi: 10.1016/j.bbrep.2021.100920. eCollection 2021 Mar. Biochem Biophys Rep. 2021. PMID: 33553686 Free PMC article.
-
Cell cycle delay in murine pre-osteoblasts is more pronounced after exposure to high-LET compared to low-LET radiation.Radiat Environ Biophys. 2014 Mar;53(1):73-81. doi: 10.1007/s00411-013-0499-0. Epub 2013 Nov 16. Radiat Environ Biophys. 2014. PMID: 24240273
References
-
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. - PubMed
-
- Hopyan S, Gokgoz N, Bell RS, Andrulis IL, Alman BA, Wunder JS. Expression of osteocalcin and its transcriptional regulators core-binding factor alpha 1 and MSX2 in osteoid-forming tumours. J Orthop Res. 1999;17:633–638. - PubMed
-
- Arai Y, Takeuchi H, Oishi K, Yoshida O. Osteocalcin: is it a useful marker of bone metastasis and response to treatment in advanced prostate cancer? Prostate. 1992;20:169–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

