NTS-Polyplex: a potential nanocarrier for neurotrophic therapy of Parkinson's disease
- PMID: 22406187
- PMCID: PMC3394898
- DOI: 10.1016/j.nano.2012.02.009
NTS-Polyplex: a potential nanocarrier for neurotrophic therapy of Parkinson's disease
Abstract
Nanomedicine has focused on targeted neurotrophic gene delivery to the brain as a strategy to stop and reverse neurodegeneration in Parkinson's disease. Because of improved transfection ability, synthetic nanocarriers have become candidates for neurotrophic therapy. Neurotensin (NTS)-polyplex is a "Trojan horse" synthetic nanocarrier system that enters dopaminergic neurons through NTS receptor internalization to deliver a genetic cargo. The success of preclinical studies with different neurotrophic genes supports the possibility of using NTS-polyplex in nanomedicine. In this review, we describe the mechanism of NTS-polyplex transfection. We discuss the concept that an effective neurotrophic therapy requires a simultaneous effect on the axon terminals and soma of the remaining dopaminergic neurons. We also discuss the future of this strategy for the treatment of Parkinson's disease.
From the clinical editor: This review paper focuses on nanomedicine-based treatment of Parkinson's disease, a neurodegenerative condition with existing symptomatic but no curative treatment. Neurotensin-polyplex is a synthetic nanocarrier system that enables delivery of genetic cargo to dopaminergic neurons via NTS receptor internalization.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



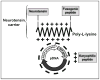





References
-
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. - PubMed
-
- Brooks DJ. PET studies on the function of dopamine in health and Parkinson’s disease. Ann N Y Acad Sci. 2003;991:22–35. - PubMed
-
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–74. - PubMed
-
- Anaya-Martinez V, Martinez-Marcos A, Martinez-Fong D, Aceves J, Erlij D. Substantia nigra compacta neurons that innervate the reticular thalamic nucleus in the rat also project to striatum or globus pallidus: implications for abnormal motor behavior. Neuroscience. 2006;143:477–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

