Strategies to defeat ketamine-induced neonatal brain injury
- PMID: 22406413
- PMCID: PMC3358446
- DOI: 10.1016/j.neuroscience.2012.02.015
Strategies to defeat ketamine-induced neonatal brain injury
Abstract
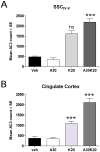
Studies using animal models have shown that general anesthetics such as ketamine trigger widespread and robust apoptosis in the infant rodent brain. Recent clinical evidence suggests that the use of general anesthetics on young children (at ages equivalent to those used in rodent studies) can promote learning deficits as they mature. Thus, there is a growing need to develop strategies to prevent this injury. In this study, we describe a number of independent approaches to address therapeutic intervention. Postnatal day 7 (P7) rats were injected with vehicle (sterile PBS) or the NMDAR antagonist ketamine (20 mg/kg). After 8 h, we prepared brains for immunohistochemical detection of the pro-apoptotic enzyme activated caspase-3 (AC3). Focusing on the somatosensory cortex, AC3-positive cells were then counted in a non-biased stereological manner. We found AC3 levels were markedly increased in ketamine-treated animals. In one study, microarray analysis of the somatosensory cortex from ketamine-treated P7 pups revealed that expression of activity dependent neuroprotective protein (ADNP) was enhanced. Thus, we injected P7 animals with the ADNP peptide fragment NAPVSIPQ (NAP) 15 min before ketamine administration and found we could dose-dependently reverse the injury. In separate studies, pretreatment of P6 animals with 20 mg/kg vitamin D(3) or a nontoxic dose of ketamine (5 mg/kg) also prevented ketamine-induced apoptosis at P7. In contrast, pretreatment of P7 animals with aspirin (30 mg/kg) 15 min before ketamine administration actually increased AC3 counts in some regions. These data show that a number of unique approaches can be taken to address anesthesia-induced neurotoxicity in the infant brain, thus providing MDs with a variety of alternative strategies that enhance therapeutic flexibility.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
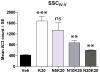
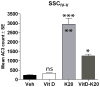
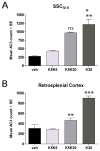
Similar articles
-
Exogenous GM1 Ganglioside Attenuates Ketamine-Induced Neurocognitive Impairment in the Developing Rat Brain.Anesth Analg. 2020 Feb;130(2):505-517. doi: 10.1213/ANE.0000000000004570. Anesth Analg. 2020. PMID: 31934908
-
Role of glycogen synthase kinase-3β in ketamine-induced developmental neuroapoptosis in rats.Br J Anaesth. 2013 Jun;110 Suppl 1:i3-9. doi: 10.1093/bja/aet057. Epub 2013 Mar 26. Br J Anaesth. 2013. PMID: 23533250
-
Baicalin Attenuates Ketamine-Induced Neurotoxicity in the Developing Rats: Involvement of PI3K/Akt and CREB/BDNF/Bcl-2 Pathways.Neurotox Res. 2016 Aug;30(2):159-72. doi: 10.1007/s12640-016-9611-y. Epub 2016 Mar 1. Neurotox Res. 2016. PMID: 26932180
-
Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system.Anesth Analg. 2008 Jun;106(6):1643-58. doi: 10.1213/ane.ob013e3181732c01. Anesth Analg. 2008. PMID: 18499593 Review.
-
Dual effects of ketamine: neurotoxicity versus neuroprotection in anesthesia for the developing brain.J Neurosurg Anesthesiol. 2014 Apr;26(2):155-60. doi: 10.1097/ANA.0000000000000027. J Neurosurg Anesthesiol. 2014. PMID: 24275940 Review.
Cited by
-
Effects of ketamine on neurogenesis, extracellular matrix homeostasis and proliferation in hypoxia-exposed HT22 murine hippocampal neurons.Biomed Rep. 2020 Oct;13(4):23. doi: 10.3892/br.2020.1330. Epub 2020 Jul 17. Biomed Rep. 2020. PMID: 32765862 Free PMC article.
-
Sex-dependent changes in ketamine-induced locomotor activity and ketamine pharmacokinetics in preweanling, adolescent, and adult rats.Eur Neuropsychopharmacol. 2019 Jun;29(6):740-755. doi: 10.1016/j.euroneuro.2019.03.013. Epub 2019 Apr 10. Eur Neuropsychopharmacol. 2019. PMID: 30981586 Free PMC article.
-
An open-label study evaluating the safety, behavioral, and electrophysiological outcomes of low-dose ketamine in children with ADNP syndrome.HGG Adv. 2022 Aug 27;3(4):100138. doi: 10.1016/j.xhgg.2022.100138. eCollection 2022 Oct 13. HGG Adv. 2022. PMID: 36119806 Free PMC article.
-
Mechanistic insights into neurotoxicity induced by anesthetics in the developing brain.Int J Mol Sci. 2012;13(6):6772-6799. doi: 10.3390/ijms13066772. Epub 2012 Jun 4. Int J Mol Sci. 2012. PMID: 22837663 Free PMC article. Review.
-
Ketamine induces hippocampal apoptosis through a mechanism associated with the caspase-1 dependent pyroptosis.Neuropharmacology. 2018 Jan;128:63-75. doi: 10.1016/j.neuropharm.2017.09.035. Epub 2017 Sep 28. Neuropharmacology. 2018. PMID: 28963039 Free PMC article.
References
-
- Asimiadou S, Bittigau P, Felderhoff-Mueser U, Manthey D, Sifringer M, Pesditschek S, Dzietko M, Kaindl AM, Pytel M, Studniarczyk D, Mozrzymas JW, Ikonomidou C. Protection with estradiol in developmental models of apoptotic neurodegeneration. Ann Neurol. 2005;58:266–276. - PubMed
-
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, Bassan H, Blat C, Gibney G, Glazner G, Brenneman DE, Gozes I. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–1293. - PubMed
-
- Berger C, Xia F, Schabitz WR, Schwab S, Grau A. High-dose aspirin is neuroprotective in a rat focal ischemia model. Brain Res. 2004;998:237–242. - PubMed
-
- Cattano D, Valleggi S, Ma D, Kastsiuchenka O, Abramo A, Sun P, Cavazzana AO, Natale G, Maze M, Giunta F. Xenon induces transcription of ADNP in neonatal rat brain. Neurosci Lett. 2008;440:217–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

