Bioencapsulation of the hepatitis B surface antigen and its use as an effective oral immunogen
- PMID: 22406456
- PMCID: PMC3322283
- DOI: 10.1016/j.vaccine.2012.02.072
Bioencapsulation of the hepatitis B surface antigen and its use as an effective oral immunogen
Abstract
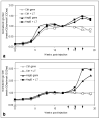
Hepatitis B remains a major global health problem despite the availability of a safe and effective vaccine. Segments of the population lack access to or respond poorly to the parenteral vaccine, perpetuating the infection-transmission cycle. A low cost, orally delivered vaccine has the potential to alleviate many of these problems. Here we describe the expression of a bioencapsulated hepatitis B surface antigen (HBsAg) in maize and its immunogenicity, demonstrating for the first time a commercially feasible oral subunit vaccine production system for a major disease. This work surmounts previous barriers to plant-produced vaccines by expressing HBsAg at much higher levels and retaining antigen immunogenicity post-processing: factors which facilitated a robust immune response in mice without the need for an adjuvant. This method provides a practical solution to the delivery of a low-cost, stable oral vaccine.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

References
-
- Ott J, Stevens G, Groeger J, Wiersma S. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012 In press( http://dx.doi.org/10.1016/j.vaccine.2011.12.116) - DOI - PubMed
-
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B Virus Infection: Epidemiology and Vaccination. Epidemiol Rev. 2006;28(1):112–25. - PubMed
-
- Stevens CE, Alter HJ, Taylor PE, Zang EA, Harley EJ, Szmuness W. Hepatitis B Vaccine in Patients Receiving Hemodialysis - Immunogenicity and Efficacy. N Engl JMed. 1984;311(8):496–501. - PubMed
-
- Roome AJ, Walsh SJ, Cartter ML, Hadler JL. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA. 1993;270(24):2931–4. - PubMed
-
- Ahishali E, Boztas G, Akyuz F, Ibrisim D, Poturoglu S, Pinarbasi B, et al. Response to Hepatitis B Vaccination in Patients with Celiac Disease. Dig Dis Sci. 2008;53(8):2156–9. - PubMed
Web references
-
- [Last accessed February 3, 2012];WHO 2010 publication. http://whqlibdoc.who.int/hq/2010/WHO_IVB_2010_eng.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

