Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells
- PMID: 22406476
- PMCID: PMC3682211
- DOI: 10.1053/j.gastro.2012.02.050
Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells
Abstract
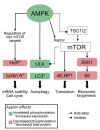
Background & aims: Aspirin reduces the incidence of and mortality from colorectal cancer (CRC) by unknown mechanisms. Cancer cells have defects in signaling via the mechanistic target of rapamycin (mTOR), which regulates proliferation. We investigated whether aspirin affects adenosine monophosphate-activated protein kinase (AMPK) and mTOR signaling in CRC cells.
Methods: The effects of aspirin on mTOR signaling, the ribosomal protein S6, S6 kinase 1 (S6K1), and eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) were examined in CRC cells by immunoblotting. Phosphorylation of AMPK was measured; the effects of loss of AMPKα on the aspirin-induced effects of mTOR were determined using small interfering RNA (siRNA) in CRC cells and in AMPK(α1/α2-/-) mouse embryonic fibroblasts. LC3 and ULK1 were used as markers of autophagy. We analyzed rectal mucosa samples from patients given 600 mg aspirin, once daily for 1 week.
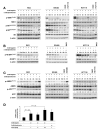
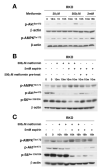
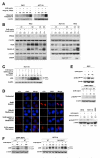
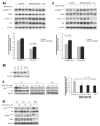
Results: Aspirin reduced mTOR signaling in CRC cells by inhibiting the mTOR effectors S6K1 and 4E-BP1. Aspirin changed nucleotide ratios and activated AMPK in CRC cells. mTOR was still inhibited by aspirin in CRC cells after siRNA knockdown of AMPKα, indicating AMPK-dependent and AMPK-independent mechanisms of aspirin-induced inhibition of mTOR. Aspirin induced autophagy, a feature of mTOR inhibition. Aspirin and metformin (an activator of AMPK) increased inhibition of mTOR and Akt, as well as autophagy in CRC cells. Rectal mucosal samples from patients given aspirin had reduced phosphorylation of S6K1 and S6.
Conclusions: Aspirin is an inhibitor of mTOR and an activator of AMPK, targeting regulators of intracellular energy homeostasis and metabolism. These could contribute to its protective effects against development of CRC.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Din FV, Theodoratou E, Farrington SM, et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59:1670–1679. - PubMed
-
- Rothwell PM, Wilson M, Elwin C-E, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. - PubMed
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

