Pancreatic ductal adenocarcinoma: a review of immunologic aspects
- PMID: 22406516
- PMCID: PMC3319488
- DOI: 10.2310/JIM.0b013e31824a4d79
Pancreatic ductal adenocarcinoma: a review of immunologic aspects
Abstract
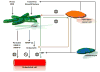
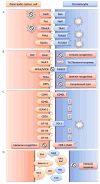
With the continued failures of both early diagnosis and treatment options for pancreatic cancer, it is now time to comprehensively evaluate the role of the immune system on the development and progression of pancreatic cancer. It is important to develop strategies that harness the molecules and cells of the immune system to treat this disease. This review will focus primarily on the role of immune cells in the development and progression of pancreatic ductal adenocarcinoma and to evaluate what is known about the interaction of immune cells with the tumor microenvironment and their role in tumor growth and metastasis. We will conclude with a brief discussion of therapy for pancreatic cancer and the potential role for immunotherapy. We hypothesize that the role of the immune system in tumor development and progression is tissue specific. Our hope is that better understanding of this process will lead to better treatments for this devastating disease.
Figures

References
-
- Emmrich J, Sparmann G, Hopt U, Lohr M, Liebe S. Typing of leukocytes in pancreatic tissue surrounding human pancreatic carcinoma. Ann N Y Acad Sci. 1999;880:171–174. - PubMed
-
- Fogar P, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–28. 00006676-200601000-00004 [pii] - PubMed
-
- Tassi E, et al. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol. 2008;181:6595–6603. 181/9/6595 [pii] - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

