A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion
- PMID: 22406518
- PMCID: PMC3359144
- DOI: 10.1038/nchembio.911
A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion
Abstract
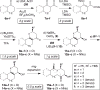
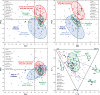
Macrocycles are key structural elements in numerous bioactive small molecules and are attractive targets in the diversity-oriented synthesis of natural product-based libraries. However, efficient and systematic access to diverse collections of macrocycles has proven difficult using classical macrocyclization reactions. To address this problem, we have developed a concise, modular approach to the diversity-oriented synthesis of macrolactones and macrolactams involving oxidative cleavage of a bridging double bond in polycyclic enol ethers and enamines. These substrates are assembled in only four or five synthetic steps and undergo ring expansion to afford highly functionalized macrocycles bearing handles for further diversification. In contrast to macrocyclization reactions of corresponding seco acids, the ring expansion reactions are efficient and insensitive to ring size and stereochemistry, overcoming key limitations of conventional approaches to systematic macrocycle synthesis. Cheminformatic analysis indicates that these macrocycles access regions of chemical space that overlap with natural products, distinct from currently targeted synthetic drugs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

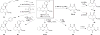
References
-
- Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery - An underexploited structural class. Nat Rev Drug Discov. 2008;7:608–624. - PubMed
-
- Wessjohann LA, Ruijter E, Garcia-Rivera D, Brandt W. What can a chemist learn from nature’s macrocycles?- A brief, conceptual view. Mol Div. 2005;9:171–186. - PubMed
-
- Sanchez-Pedregal VM, et al. The tubulin-bound conformation of discodermolide derived by NMR studies in solution supports a common pharmacophore model for epothilone and discodermolide. Angew Chem, Int Ed. 2006;45:7388–7394. - PubMed
-
- Canales A, et al. The bound conformation of microtubule-stabilizing agents: NMR insights into the bioactive 3D structure of discodermolide and dictyostatin. Chem Eur J. 2008;14:7557–7569. - PubMed
-
- Knust H, Hoffmann RW. Synthesis and conformational analysis of macrocyclic dilactones mimicking the pharmacophore of aplysiatoxin. Helv Chim Acta. 2003;86:1871–1893.
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/134337221
- PubChem-Substance/134337222
- PubChem-Substance/134337223
- PubChem-Substance/134337224
- PubChem-Substance/134337225
- PubChem-Substance/134337226
- PubChem-Substance/134337227
- PubChem-Substance/134337228
- PubChem-Substance/134337229
- PubChem-Substance/134337230
- PubChem-Substance/134337231
- PubChem-Substance/134337232
- PubChem-Substance/134337233
- PubChem-Substance/134337234
- PubChem-Substance/134337235
- PubChem-Substance/134337236
- PubChem-Substance/134337237
- PubChem-Substance/134337238
- PubChem-Substance/134337239
- PubChem-Substance/134337240
- PubChem-Substance/134337241
- PubChem-Substance/134337242
- PubChem-Substance/134337243
- PubChem-Substance/134337244
- PubChem-Substance/134337245
- PubChem-Substance/134337246
- PubChem-Substance/134337247
- PubChem-Substance/134337248
- PubChem-Substance/134337249
- PubChem-Substance/134337250
- PubChem-Substance/134337251
- PubChem-Substance/134337252
- PubChem-Substance/134337253
- PubChem-Substance/134337254
- PubChem-Substance/134337255
- PubChem-Substance/134337256
- PubChem-Substance/134337257
- PubChem-Substance/134337258
- PubChem-Substance/134337259
- PubChem-Substance/134337260
- PubChem-Substance/134337261
- PubChem-Substance/134337262
- PubChem-Substance/134337263
- PubChem-Substance/134337264
- PubChem-Substance/134337265
- PubChem-Substance/134337266
- PubChem-Substance/134337267
- PubChem-Substance/134337268
- PubChem-Substance/134337269
- PubChem-Substance/134337270
- PubChem-Substance/134337271
- PubChem-Substance/134337272
- PubChem-Substance/134337273
- PubChem-Substance/134337274
- PubChem-Substance/134337275
- PubChem-Substance/134337276
- PubChem-Substance/134337277
- PubChem-Substance/134337278
- PubChem-Substance/134337279
- PubChem-Substance/134337280
- PubChem-Substance/134337281
- PubChem-Substance/134337282
- PubChem-Substance/134337283
- PubChem-Substance/134337284
- PubChem-Substance/134337285
- PubChem-Substance/134337286
- PubChem-Substance/134337287
- PubChem-Substance/134337288
- PubChem-Substance/134337289
- PubChem-Substance/134337290
- PubChem-Substance/134337291
- PubChem-Substance/134337292
- PubChem-Substance/134337293
- PubChem-Substance/134337294
- PubChem-Substance/134337295
- PubChem-Substance/134337296
- PubChem-Substance/134337297
- PubChem-Substance/134337298
- PubChem-Substance/134337299
- PubChem-Substance/134337300
- PubChem-Substance/134337301
- PubChem-Substance/134337302
- PubChem-Substance/134337303
- PubChem-Substance/134337304
- PubChem-Substance/134337305
- PubChem-Substance/134337306
- PubChem-Substance/134337307
- PubChem-Substance/134337308
- PubChem-Substance/134337309
- PubChem-Substance/134337310
- PubChem-Substance/134337311
- PubChem-Substance/134337312
- PubChem-Substance/134337313
- PubChem-Substance/134337314
- PubChem-Substance/134337315
- PubChem-Substance/134337316
- PubChem-Substance/134337317
- PubChem-Substance/134337318
- PubChem-Substance/134337319
- PubChem-Substance/134337320
- PubChem-Substance/134337321
- PubChem-Substance/134337322
- PubChem-Substance/134337323
- PubChem-Substance/134337324
- PubChem-Substance/134337325
- PubChem-Substance/134337326
- PubChem-Substance/134337327
- PubChem-Substance/134337328
- PubChem-Substance/134337329
- PubChem-Substance/134337330
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

