S-glutathionylated serine proteinase inhibitors as plasma biomarkers in assessing response to redox-modulating drugs
- PMID: 22406622
- PMCID: PMC4151169
- DOI: 10.1158/0008-5472.CAN-11-4088
S-glutathionylated serine proteinase inhibitors as plasma biomarkers in assessing response to redox-modulating drugs
Abstract
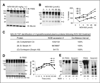
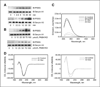
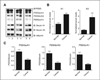
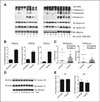
Many cancer drugs impact cancer cell redox regulatory mechanisms and disrupt redox homeostasis. Pharmacodynamic biomarkers that measure therapeutic efficacy or toxicity could improve patient management. Using immunoblot analyses and mass spectrometry, we identified that serpins A1 and A3 were S-glutathionylated in a dose- and time-dependent manner following treatment of mice with drugs that alter reactive oxygen or nitrogen species. Tandem mass spectrometry analyses identified Cys(256) of serpin A1 and Cys(263) of serpin A3 as the S-glutathionylated residues. In human plasma from cancer patients, there were higher levels of unmodified serpin A1 and A3, but following treatments with redox active drugs, relative S-glutathionylation of these serpins was higher in plasma from normal individuals. There is potential for S-glutathionylated serpins A1 and A3 to act as pharmacodynamic biomarkers for evaluation of patient response to drugs that target redox pathways.
©2012 AACR
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
S-Glutathionylated Serine Proteinase Inhibitors as Biomarkers for Radiation Exposure in Prostate Cancer Patients.Sci Rep. 2019 Sep 24;9(1):13792. doi: 10.1038/s41598-019-50288-9. Sci Rep. 2019. PMID: 31551460 Free PMC article.
-
Glutathionylation of trypanosomal thiol redox proteins.J Biol Chem. 2007 Mar 23;282(12):8678-94. doi: 10.1074/jbc.M608140200. Epub 2007 Jan 22. J Biol Chem. 2007. PMID: 17242409
-
Analysis of the plasma elimination kinetics and conformational stabilities of native, proteinase-complexed, and reactive site cleaved serpins: comparison of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, antithrombin III, alpha 2-antiplasmin, angiotensinogen, and ovalbumin.Biochemistry. 1991 Feb 12;30(6):1723-30. doi: 10.1021/bi00220a039. Biochemistry. 1991. PMID: 1704258
-
Serine proteinase inhibitors as acute phase reactants in liver disease.Tokai J Exp Clin Med. 1988 Dec;13(6):365-71. Tokai J Exp Clin Med. 1988. PMID: 2483770 Review.
-
The functional role of acute phase plasma proteinase inhibitors.Tokai J Exp Clin Med. 1988 Dec;13(6):313-20. Tokai J Exp Clin Med. 1988. PMID: 3152555 Review.
Cited by
-
S-Glutathionylated Serine Proteinase Inhibitors as Biomarkers for Radiation Exposure in Prostate Cancer Patients.Sci Rep. 2019 Sep 24;9(1):13792. doi: 10.1038/s41598-019-50288-9. Sci Rep. 2019. PMID: 31551460 Free PMC article.
-
Ultramicronized N-Palmitoylethanolamine Regulates Mast Cell-Astrocyte Crosstalk: A New Potential Mechanism Underlying the Inhibition of Morphine Tolerance.Biomolecules. 2023 Jan 25;13(2):233. doi: 10.3390/biom13020233. Biomolecules. 2023. PMID: 36830602 Free PMC article.
-
S-glutathionylation of buccal cell proteins as biomarkers of exposure to hydrogen peroxide.BBA Clin. 2014 Sep 3;2:31-9. doi: 10.1016/j.bbacli.2014.08.003. eCollection 2014 Dec. BBA Clin. 2014. PMID: 26673080 Free PMC article.
-
Adaptations in glutathione-based redox protein signaling pathways and alcohol drinking across species.Biomed Pharmacother. 2024 Nov;180:117514. doi: 10.1016/j.biopha.2024.117514. Epub 2024 Oct 2. Biomed Pharmacother. 2024. PMID: 39362067 Free PMC article.
-
Glutathione S-Transferases in Cancer.Antioxidants (Basel). 2021 Apr 29;10(5):701. doi: 10.3390/antiox10050701. Antioxidants (Basel). 2021. PMID: 33946704 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

