Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy
- PMID: 22407289
- PMCID: PMC3326159
- DOI: 10.1208/s12248-012-9340-y
Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy
Abstract
The development of therapeutic proteins requires the understanding of the relationship between the dose, exposure, efficacy, and toxicity of these molecules. Several intrinsic and extrinsic factors contribute to the challenges for measuring therapeutic proteins in a precise and accurate manner. In addition, induction of an immune response to therapeutic protein results in additional complexities in the analysis of the pharmacokinetic profile, toxicity, safety, and efficacy of this class of molecules. Assessment of immunogenicity of therapeutic proteins is a required aspect of regulatory filings for a licensing application and for the safe and efficacious use of these compounds. A systematic strategy and well-defined criteria for measuring anti-drug antibodies (ADA) have been established, to a large extent, through coordinated efforts. These recommendations are based on risk assessment and include the determination of ADA content (concentration/titer), affinity, immunoglobulin isotype/subtype, and neutralization capacity. This manuscript reviews the requirements necessary for understanding the nature of an ADA response in order to discern the impact of immunogenicity on pharmacokinetics/pharmacodynamics and efficacy.
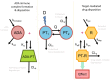
Figures
References
-
- Meibohm B, Braeckman RA. Pharmacokinetics and pharmacodynamics of peptides and proteins. In: Crommelin DJA, Sindelar RD, Meibohm B, editors. Pharmaceutical biotechnology: concepts and applications. 3. New York: Informa Healthcare; 2007. pp. 95–123.
-
- Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, et al. Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet. 2011;50:131–142. doi: 10.2165/11537430-000000000-00000. - DOI - PubMed
-
- CHMP. Guideline of immunogenicity assessment of biotechnology-derived therapeutic proteins. London: EMEA Guidance Document; 2008.
-
- Gupta S, Devanarayan V, Finco D, Gunn GR, 3rd, Kirshner S, Richards S, et al. Recommendations for the validation of cell-based assays used for the detection of neutralizing antibody immune responses elicited against biological therapeutics. J Pharm Biomed Anal. 2011;55:878–888. doi: 10.1016/j.jpba.2011.03.038. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

