Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling
- PMID: 22407318
- PMCID: PMC3885420
- DOI: 10.1038/nature10893
Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling
Abstract
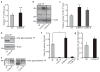
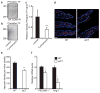
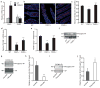
The gut microbiota is a complex ecosystem that has coevolved with host physiology. Colonization of germ-free (GF) mice with a microbiota promotes increased vessel density in the small intestine, but little is known about the mechanisms involved. Tissue factor (TF) is the membrane receptor that initiates the extrinsic coagulation pathway, and it promotes developmental and tumour angiogenesis. Here we show that the gut microbiota promotes TF glycosylation associated with localization of TF on the cell surface, the activation of coagulation proteases, and phosphorylation of the TF cytoplasmic domain in the small intestine. Anti-TF treatment of colonized GF mice decreased microbiota-induced vascular remodelling and expression of the proangiogenic factor angiopoietin-1 (Ang-1) in the small intestine. Mice with a genetic deletion of the TF cytoplasmic domain or with hypomorphic TF (F3) alleles had a decreased intestinal vessel density. Coagulation proteases downstream of TF activate protease-activated receptor (PAR) signalling implicated in angiogenesis. Vessel density and phosphorylation of the cytoplasmic domain of TF were decreased in small intestine from PAR1-deficient (F2r(-/-)) but not PAR2-deficient (F2rl1(-/-)) mice, and inhibition of thrombin showed that thrombin-PAR1 signalling was upstream of TF phosphorylation. Thus, the microbiota-induced extravascular TF-PAR1 signalling loop is a novel pathway that may be modulated to influence vascular remodelling in the small intestine.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Morrissey JH, Fakhrai H, Edgington TS. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987;50:129–135. - PubMed
-
- Carmeliet P, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. - PubMed
-
- Belting M, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nature Med. 2004;10:502–509. - PubMed
-
- Griffin TC, Srinivasan Y, Zheng YW, Huang W, Coughlin SR. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293:1666–1670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

