S-adenosyl methionine regulates ubiquitin-conjugating enzyme 9 protein expression and sumoylation in murine liver and human cancers
- PMID: 22407595
- PMCID: PMC3378793
- DOI: 10.1002/hep.25701
S-adenosyl methionine regulates ubiquitin-conjugating enzyme 9 protein expression and sumoylation in murine liver and human cancers
Abstract
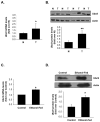
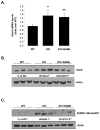
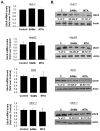
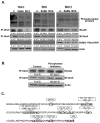
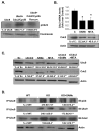
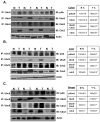
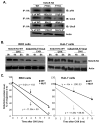
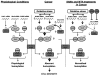
Ubiquitin-conjugating enzyme 9 (Ubc9) is required for sumoylation and is overexpressed in several malignancies, but its expression in hepatocellular carcinoma (HCC) is unknown. Hepatic S-adenosyl methionine (SAMe) levels decrease in methionine adenosyltransferase 1A (Mat1a) knockout (KO) mice, which develop HCC, and in ethanol-fed mice. We examined the regulation of Ubc9 by SAMe in murine liver and human HCC, breast, and colon carcinoma cell lines and specimens. Real-time polymerase chain reaction and western blotting measured gene and protein expression, respectively. Immunoprecipitation followed by western blotting examined protein-protein interactions. Ubc9 expression increased in HCC and when hepatic SAMe levels decreased. SAMe treatment in Mat1a KO mice reduced Ubc9 protein, but not messenger RNA (mRNA) levels, and lowered sumoylation. Similarly, treatment of liver cancer cell lines HepG2 and Huh7, colon cancer cell line RKO, and breast cancer cell line MCF-7 with SAMe or its metabolite 5'-methylthioadenosine (MTA) reduced only Ubc9 protein level. Ubc9 posttranslational regulation is unknown. Ubc9 sequence predicted a possible phosphorylation site by cell division cycle 2 (Cdc2), which directly phosphorylated recombinant Ubc9. Mat1a KO mice had higher phosphorylated (phospho)-Ubc9 levels, which normalized after SAMe treatment. SAMe and MTA treatment lowered Cdc2 mRNA and protein levels, as well as phospho-Ubc9 and protein sumoylation in liver, colon, and breast cancer cells. Serine 71 of Ubc9 was required for phosphorylation, interaction with Cdc2, and protein stability. Cdc2, Ubc9, and phospho-Ubc9 levels increased in human liver, breast, and colon cancers.
Conclusion: Cdc2 expression is increased and Ubc9 is hyperphosphorylated in several cancers, and this represents a novel mechanism to maintain high Ubc9 protein expression that can be inhibited by SAMe and MTA.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures
Similar articles
-
Methionine adenosyltransferase α2 sumoylation positively regulate Bcl-2 expression in human colon and liver cancer cells.Oncotarget. 2015 Nov 10;6(35):37706-23. doi: 10.18632/oncotarget.5342. Oncotarget. 2015. PMID: 26416353 Free PMC article.
-
Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis.J Hepatol. 2013 Oct;59(4):830-41. doi: 10.1016/j.jhep.2013.04.031. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665184 Review.
-
S-adenosylmethionine inhibits la ribonucleoprotein domain family member 1 in murine liver and human liver cancer cells.Hepatology. 2022 Feb;75(2):280-296. doi: 10.1002/hep.32130. Epub 2021 Dec 18. Hepatology. 2022. PMID: 34449924 Free PMC article.
-
Targeting UBC9-mediated protein hyper-SUMOylation in cystic cholangiocytes halts polycystic liver disease in experimental models.J Hepatol. 2021 Feb;74(2):394-406. doi: 10.1016/j.jhep.2020.09.010. Epub 2020 Sep 17. J Hepatol. 2021. PMID: 32950589 Free PMC article.
-
Targeting Ubc9 for cancer therapy.Expert Opin Ther Targets. 2005 Dec;9(6):1203-16. doi: 10.1517/14728222.9.6.1203. Expert Opin Ther Targets. 2005. PMID: 16300471 Review.
Cited by
-
Aberrant post-translational protein modifications in the pathogenesis of alcohol-induced liver injury.World J Gastroenterol. 2016 Jul 21;22(27):6192-200. doi: 10.3748/wjg.v22.i27.6192. World J Gastroenterol. 2016. PMID: 27468209 Free PMC article. Review.
-
New insights on the role of epigenetic alterations in hepatocellular carcinoma.J Hepatocell Carcinoma. 2014 Jun 12;1:65-83. doi: 10.2147/JHC.S44506. eCollection 2014. J Hepatocell Carcinoma. 2014. PMID: 27508177 Free PMC article. Review.
-
Alterations of Methionine Metabolism as Potential Targets for the Prevention and Therapy of Hepatocellular Carcinoma.Medicina (Kaunas). 2019 Jun 21;55(6):296. doi: 10.3390/medicina55060296. Medicina (Kaunas). 2019. PMID: 31234428 Free PMC article. Review.
-
SUMO and the robustness of cancer.Nat Rev Cancer. 2017 Mar;17(3):184-197. doi: 10.1038/nrc.2016.143. Epub 2017 Jan 30. Nat Rev Cancer. 2017. PMID: 28134258 Review.
-
Contributing roles of mitochondrial dysfunction and hepatocyte apoptosis in liver diseases through oxidative stress, post-translational modifications, inflammation, and intestinal barrier dysfunction.Cell Mol Life Sci. 2024 Jan 12;81(1):34. doi: 10.1007/s00018-023-05061-7. Cell Mol Life Sci. 2024. PMID: 38214802 Free PMC article. Review.
References
-
- Johnson ES. Protein modification by SUMO. Ann Rev Biochem. 2004;73:355–382. - PubMed
-
- Muller S, Hoege C, Pyrowalakis G, Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202–210. - PubMed
-
- Johnson ES, Blogel G. Ubc9 is the conjugating enzyme for the ubiquitin-like protein Smt3p. J Biol Chem. 1997;272:26799–26702. - PubMed
-
- Manza LL, Codreanu SG, Stamer SL, Smith DL, Wells KS, Roberts RL, et al. Global shifts in protein sumoylation in response to electrophile and oxidative stress. Chem Res Toxicol. 2004;17:1706–1715. - PubMed
-
- Romanenko AM, Kinoshita A, Wanibuchi H, Wei M, Zaparin WK, Vinnichenko WI, et al. Involvement of ubiquitination and sumoylation in bladder lesions induced by persistent long-term low dose ionizing radiation in humans. J Urol. 2006;175:739–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
