Stem-like tumor-initiating cells isolated from IL13Rα2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells
- PMID: 22407828
- PMCID: PMC3578382
- DOI: 10.1158/1078-0432.CCR-11-1669
Stem-like tumor-initiating cells isolated from IL13Rα2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells
Abstract
Purpose: To evaluate IL13Rα2 as an immunotherapeutic target for eliminating glioma stem-like cancer initiating cells (GSC) of high-grade gliomas, with particular focus on the potential of genetically engineered IL13Rα2-specific primary human CD8(+) CTLs (IL13-zetakine(+) CTL) to target this therapeutically resistant glioma subpopulation.
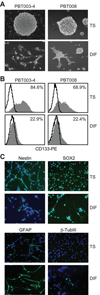
Experimental design: A panel of low-passage GSC tumor sphere (TS) and serum-differentiated glioma lines were expanded from patient glioblastoma specimens. These glioblastoma lines were evaluated for expression of IL13Rα2 and for susceptibility to IL13-zetakine(+) CTL-mediated killing in vitro and in vivo.
Results: We observed that although glioma IL13Rα2 expression varies between patients, for IL13Rα2(pos) cases this antigen was detected on both GSCs and more differentiated tumor cell populations. IL13-zetakine(+) CTL were capable of efficient recognition and killing of both IL13Rα2(pos) GSCs and IL13Rα2(pos) differentiated cells in vitro, as well as eliminating glioma-initiating activity in an orthotopic mouse tumor model. Furthermore, intracranial administration of IL13-zetakine(+) CTL displayed robust antitumor activity against established IL13Rα2(pos) GSC TS-initiated orthotopic tumors in mice.
Conclusions: Within IL13Rα2 expressing high-grade gliomas, this receptor is expressed by GSCs and differentiated tumor populations, rendering both targetable by IL13-zetakine(+) CTLs. Thus, our results support the potential usefullness of IL13Rα2-directed immunotherapeutic approaches for eradicating therapeutically resistant GSC populations.
©2012 AACR.
Conflict of interest statement
MCJ is an inventor of licensed patents and equity holder in ZetaRx, Inc a licensee of these patents. Other authors have no conflicts of interest to disclose.
Figures




References
-
- Dirks PB. Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol. 2008;26:2916–2924. - PubMed
-
- Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

