Lung development and the host response to influenza A virus are altered by different doses of neonatal oxygen in mice
- PMID: 22408042
- PMCID: PMC3362262
- DOI: 10.1152/ajplung.00026.2012
Lung development and the host response to influenza A virus are altered by different doses of neonatal oxygen in mice
Abstract
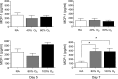
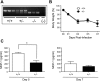
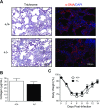
Oxygen exposure in preterm infants has been associated with altered lung development and increased risk for respiratory viral infections later in life. Although the dose of oxygen sufficient to exert these changes in humans remains unknown, adult mice exposed to 100% oxygen between postnatal days 1-4 exhibit alveolar simplification and increased sensitivity to influenza virus infection. Additionally, two nonlinear thresholds of neonatal oxygen exposures were previously identified that promote modest (between 40% and 60% oxygen) and severe (between 80% and 100% oxygen) changes in lung development. Here, we investigate whether these two thresholds correlate with the severity of lung disease following respiratory viral infection. Adult mice exposed to 100% oxygen at birth, and to a lesser extent 80% oxygen, demonstrated enhanced body weight loss, persistent inflammation, and fibrosis following infection compared with infected siblings exposed to room air at birth. In contrast, the host response to infection was indistinguishable between mice exposed to room air and 40% or 60% oxygen. Interestingly, levels of monocyte chemoattractant protein (MCP)-1 were equivalently elevated in infected mice that had been exposed to 80% or 100% oxygen as neonates. However, reducing levels of MCP-1 using heterozygous Mcp-1 mice did not affect oxygen-dependent changes in the response to infection. Thus lung development and the host response to respiratory viral infection are disrupted by different doses of oxygen. Our findings suggest that measuring lung function alone may not be sufficient to identify individuals born prematurely who have increased risk for respiratory viral infection.
Figures




References
-
- Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med 349: 959–967, 2003. - PubMed
-
- Bachmann MF, Ecabert B, Kopf M. Influenza virus: a novel method to assess viral and neutralizing antibody titers in vitro. J Immunol Methods 225: 105–111, 1999. - PubMed
-
- Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, Yoder BA, Faix RG, Das A, Poole WK, Schibler K, Newman NS, Ambalavanan N, Frantz ID, 3rd, Piazza AJ, Sanchez PJ, Morris BH, Laroia N, Phelps DL, Poindexter BB, Cotten CM, Van Meurs KP, Duara S, Narendran V, Sood BG, O'Shea TM, Bell EF, Ehrenkranz RA, Watterberg KL, Higgins RD. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med 362: 1959–1969, 2010. - PMC - PubMed
-
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PM, Celli BR, Jones PW, Mahler DA, Make B, Miravitlles M, Page CP, Palange P, Parr D, Pistolesi M, Rennard SI, Rutten-van Molken MP, Stockley R, Sullivan SD, Wedzicha JA, Wouters EF. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 31: 416–469, 2008. - PubMed
-
- Coates AL, Desmond K, Willis D, Nogrady MB. Oxygen therapy and long-term pulmonary outcome of respiratory distress syndrome in newborns. Am J Dis Child 136: 892–895, 1982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

