Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats
- PMID: 22408173
- PMCID: PMC3339639
- DOI: 10.1210/en.2011-1783
Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats
Abstract
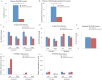
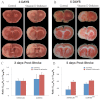
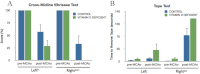
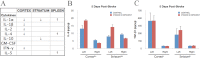
Vitamin D deficiency (VDD) is widespread and considered a risk factor for cardiovascular disease and stroke. Low vitamin D levels are predictive for stroke and more fatal strokes in humans, whereas vitamin D supplements are associated with decreased risk of all-cause mortality. Because VDD occurs with other comorbid conditions that are also independent risk factors for stroke, this study examined the specific effect of VDD on stroke severity in rats. Adult female rats were fed control or VDD diet for 8 wk and were subject to middle cerebral artery occlusion thereafter. The VDD diet reduced circulating vitamin D levels to one fifth (22%) of that observed in rats fed control chow. Cortical and striatal infarct volumes in animals fed VDD diet were significantly larger, and sensorimotor behavioral testing indicated that VDD animals had more severe poststroke behavioral impairment than controls. VDD animals were also found to have significantly lower levels of the neuroprotective hormone IGF-I in plasma and the ischemic hemisphere. Cytokine analysis indicated that VDD significantly reduced IL-1α, IL-1β, IL-2, IL-4, IFN-γ, and IL-10 expression in ischemic brain tissue. However, ischemia-induced IL-6 up-regulation was significantly higher in VDD animals. In a separate experiment, the therapeutic potential of acute vitamin D treatments was evaluated, where animals received vitamin D injections 4 h after stroke and every 24 h thereafter. Acute vitamin D treatment did not improve infarct volume or behavioral performance. Our data indicate that VDD exacerbates stroke severity, involving both a dysregulation of the inflammatory response as well as suppression of known neuroprotectants such as IGF-I.
Figures

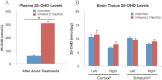
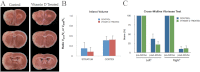
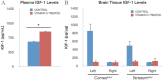
References
-
- Baeke F, Gysemans C, Korf H, Mathieu C. 2010. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol 25:1597–1606 - PubMed
-
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. 2007. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647 - PubMed
-
- Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, Bartik L, Egan JB, Wu Y, Kubicek JL, Lowmiller CL, Moffet EW, Forster RE, Jurutka PW. 2010. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “fountain of youth” to mediate healthful aging. J Steroid Biochem Mol Biol 121:88–97 - PMC - PubMed
-
- Bikle DD. 2009. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep 7:58–63 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

