The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters
- PMID: 22408259
- PMCID: PMC3359736
- DOI: 10.1182/blood-2011-09-381970
The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters
Abstract
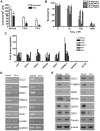
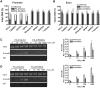
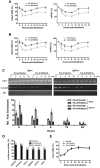
Oxidative stress has been implicated in the pathogenesis of many human diseases including Fanconi anemia (FA), a genetic disorder associated with BM failure and cancer. Here we show that major antioxidant defense genes are down-regulated in FA patients, and that gene down-regulation is selectively associated with increased oxidative DNA damage in the promoters of the antioxidant defense genes. Assessment of promoter activity and DNA damage repair kinetics shows that increased initial damage, rather than a reduced repair rate, contributes to the augmented oxidative DNA damage. Mechanistically, FA proteins act in concert with the chromatin-remodeling factor BRG1 to protect the promoters of antioxidant defense genes from oxidative damage. Specifically, BRG1 binds to the promoters of the antioxidant defense genes at steady state. On challenge with oxidative stress, FA proteins are recruited to promoter DNA, which correlates with significant increase in the binding of BRG1 within promoter regions. In addition, oxidative stress-induced FANCD2 ubiquitination is required for the formation of a FA-BRG1-promoter complex. Taken together, these data identify a role for the FA pathway in cellular antioxidant defense.
Figures



References
-
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38(1):96–109. - PubMed
-
- Weiss JM, Goode EL, Ladiges WC, Ulrich CM. Polymorphic variation in hOGG1 and risk of cancer: A review of the functional and epidemiologic literature. Mol Carcinog. 2005;42(3):127–141. - PubMed
-
- Gottschling BC, Maronpot RR, Hailey JR, et al. The role of oxidative stress in indium phosphide-induced lung carcinogenesis in rats. Toxicol Sci. 2001;64(1):28–40. - PubMed
-
- Muguruma M, Unami A, Kanki M, et al. Possible involvement of oxidative stress in piperonyl butoxide induced hepatocarcinogenesis in rats. Toxicology. 2007;236(1-2):61–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

