Dissociation Behavior of Tryptic and Intramolecular Disulfide-linked Peptide Ions Modified in the Gas Phase via Ion/Ion Reactions
- PMID: 22408389
- PMCID: PMC3297198
- DOI: 10.1016/j.ijms.2011.07.002
Dissociation Behavior of Tryptic and Intramolecular Disulfide-linked Peptide Ions Modified in the Gas Phase via Ion/Ion Reactions
Abstract
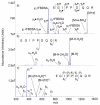
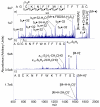
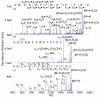
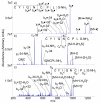
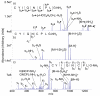
Protonated tryptic peptides, somatostatin-14, and oxytocin have been subjected to reactions with doubly deprotonated 4-formyl-1,3-benzenedisulfonic acid (FBDSA) in the gas phase. The major product is a negatively-charged complex comprised of the peptide and the reagent. Upon dehydration of the complex, all peptides show evidence for Schiff base formation involving a primary amine of the peptide. Some peptides also show evidence for the formation of a relatively strong electrostatic interaction without Schiff base formation (i.e., a mixture of isomeric precursor ions is generated upon dehydration of the complex). Ion trap collision-induced dissociation of the dehydration products from all peptides examined gave distinct product ion spectra relative to the deprotonated and protonated forms of the peptides. The distinct behavior of the modified ions is attributed to the highly stable charge carrying sulfonate group, which tends to inhibit intramolecular proton transfer in negatively charged species. Modified anions of the peptides with an intramolecular disulfide linkage show evidence for cleavage of both the disulfide linkage and an amide bond in the loop defined by the disulfide bond. Modification of protonated peptides via charge inversion with FBDSA is a useful means for generating novel and distinct ion-types that can provide complementary structural information upon subsequent activation to that obtained from dissociation of protonated or deprotonated forms of the peptide.
Figures

References
-
- Stewart NA, Pham VT, Choma CT, Kaplan H. Improved Peptide Detection with Matriz-assisted Laser Desorption Ionization Mass Spectrometry by Trimethylation of Amino Groups. Rapid Commun. Mass Spectrom. 2002;16:1448–1453. - PubMed
-
- Ross PL, Huang YLN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed Protein Quantitation in Saccharomyces Cerevisiae Using Amine-reactive Isobaric Tagging Reagents. Molec. Cellular Proteomics. 2004;3:1154–1169. - PubMed
-
- Zubarev RA. Reactions of Polypeptide Ions with Electrons in the Gas Phase. Mass Spectrom. Rev. 2003;22:57–77. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
