Tumor heterogeneity: mechanisms and bases for a reliable application of molecular marker design
- PMID: 22408433
- PMCID: PMC3292002
- DOI: 10.3390/ijms13021951
Tumor heterogeneity: mechanisms and bases for a reliable application of molecular marker design
Abstract
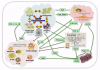
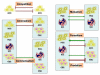
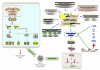
Tumor heterogeneity is a confusing finding in the assessment of neoplasms, potentially resulting in inaccurate diagnostic, prognostic and predictive tests. This tumor heterogeneity is not always a random and unpredictable phenomenon, whose knowledge helps designing better tests. The biologic reasons for this intratumoral heterogeneity would then be important to understand both the natural history of neoplasms and the selection of test samples for reliable analysis. The main factors contributing to intratumoral heterogeneity inducing gene abnormalities or modifying its expression include: the gradient ischemic level within neoplasms, the action of tumor microenvironment (bidirectional interaction between tumor cells and stroma), mechanisms of intercellular transference of genetic information (exosomes), and differential mechanisms of sequence-independent modifications of genetic material and proteins. The intratumoral heterogeneity is at the origin of tumor progression and it is also the byproduct of the selection process during progression. Any analysis of heterogeneity mechanisms must be integrated within the process of segregation of genetic changes in tumor cells during the clonal expansion and progression of neoplasms. The evaluation of these mechanisms must also consider the redundancy and pleiotropism of molecular pathways, for which appropriate surrogate markers would support the presence or not of heterogeneous genetics and the main mechanisms responsible. This knowledge would constitute a solid scientific background for future therapeutic planning.
Keywords: cell segregation; clonal expansion; exosome; metastasis; neoplasm; topographic compartments; tumor heterogeneity; tumor hypoxia; tumor microenvironment; tumor progression.
Figures

References
-
- Fidler I.J., Hart I.R. Biological diversity in metastatic neoplasms: Origins and implications. Science. 1982;217:998–1003. - PubMed
-
- Heppner G.H., Miller F.R. The cellular basis of tumor progression. Int. Rev. Cytol. 1998;177:1–56. - PubMed
-
- Talmadge J.E., Wolman S.R., Fidler I.J. Evidence for the clonal origin of spontaneous metastases. Science. 1982;217:361–363. - PubMed
-
- Diaz-Cano S.J. General morphological and biological features of neoplasms: Integration of molecular findings. Histopathology. 2008;53:1–19. - PubMed
-
- Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

