Characterization of different functionalized lipidic nanocapsules as potential drug carriers
- PMID: 22408461
- PMCID: PMC3292030
- DOI: 10.3390/ijms13022405
Characterization of different functionalized lipidic nanocapsules as potential drug carriers
Abstract
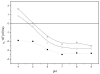
Lipid nanocapsules (LNC) based on a core-shell structure consisting of an oil-filled core with a surrounding polymer layer are known to be promising vehicles for the delivery of hydrophobic drugs in the new therapeutic strategies in anti-cancer treatments. The present work has been designed as basic research about different LNC systems. We have synthesized-and physico-chemically characterized-three different LNC systems in which the core was constituted by olive oil and the shell by different phospholipids (phosphatidyl-serine or lecithin) and other biocompatible molecules such as Pluronic(®) F68 or chitosan. It is notable that the olive-oil-phosphatidyl-serine LCN is a novel formulation presented in this work and was designed to generate an enriched carboxylic surface. This carboxylic layer is meant to link specific antibodies, which could facilitate the specific nanocapsule uptake by cancer cells. This is why nanoparticles with phosphatidyl-serine in their shell have also been used in this work to form immuno-nanocapsules containing a polyclonal IgG against a model antigen (C-reactive protein) covalently bounded by means of a simple and reproducible carbodiimide method. An immunological study was made to verify that these IgG-LNC complexes showed the expected specific immune response. Finally, a preliminary in vitro study was performed by culturing a breast-carcinoma cell line (MCF-7) with Nile-Red-loaded LNC. We found that these cancer cells take up the fluorescent Nile- Red molecule in a process dependent on the surface properties of the nanocarriers.
Keywords: drug delivery; immuno-nanocapsules; lipid nanocapsules; nanocarriers.
Figures







Similar articles
-
Synthesis and characterization of lipid immuno-nanocapsules for directed drug delivery: selective antitumor activity against HER2 positive breast-cancer cells.Biomacromolecules. 2013 Dec 9;14(12):4248-59. doi: 10.1021/bm401103t. Epub 2013 Nov 4. Biomacromolecules. 2013. PMID: 24134122
-
Radiolabeling and targeting of lipidic nanocapsules for applications in radioimmunotherapy.Q J Nucl Med Mol Imaging. 2007 Mar;51(1):51-60. Q J Nucl Med Mol Imaging. 2007. PMID: 17372573
-
Development and Evaluation of Artesunate-Loaded Chitosan-Coated Lipid Nanocapsule as a Potential Drug Delivery System Against Breast Cancer.AAPS PharmSciTech. 2015 Dec;16(6):1307-16. doi: 10.1208/s12249-015-0311-3. Epub 2015 Mar 19. AAPS PharmSciTech. 2015. PMID: 25787869 Free PMC article.
-
Lipid Nanocapsule: A Novel Approach to Drug Delivery System Formulation Development.Curr Pharm Biotechnol. 2024;25(3):268-284. doi: 10.2174/1389201024666230523114350. Curr Pharm Biotechnol. 2024. PMID: 37231750 Review.
-
Core-Shell Type Lipidic and Polymeric Nanocapsules: the Transformative Multifaceted Delivery Systems.AAPS PharmSciTech. 2023 Jan 26;24(1):50. doi: 10.1208/s12249-023-02504-z. AAPS PharmSciTech. 2023. PMID: 36703085 Review.
Cited by
-
Chemical and colloidal stability of carboxylated core-shell magnetite nanoparticles designed for biomedical applications.Int J Mol Sci. 2013 Jul 12;14(7):14550-74. doi: 10.3390/ijms140714550. Int J Mol Sci. 2013. PMID: 23857054 Free PMC article.
-
Exploring the Impact of Nanoparticle Stealth Coatings in Cancer Models: From PEGylation to Cell Membrane-Coating Nanotechnology.ACS Appl Mater Interfaces. 2024 Jan 17;16(2):2058-2074. doi: 10.1021/acsami.3c13948. Epub 2023 Dec 30. ACS Appl Mater Interfaces. 2024. PMID: 38159050 Free PMC article.
-
Mesenchymal stem cells-derived exosomes: novel carriers for nanoparticle to combat cancer.Eur J Med Res. 2023 Dec 9;28(1):579. doi: 10.1186/s40001-023-01556-y. Eur J Med Res. 2023. PMID: 38071346 Free PMC article. Review.
-
Applications of Nanomaterials in Leishmaniasis: A Focus on Recent Advances and Challenges.Nanomaterials (Basel). 2019 Dec 9;9(12):1749. doi: 10.3390/nano9121749. Nanomaterials (Basel). 2019. PMID: 31818029 Free PMC article. Review.
-
Immune cell impact of three differently coated lipid nanocapsules: pluronic, chitosan and polyethylene glycol.Sci Rep. 2016 Jan 5;6:18423. doi: 10.1038/srep18423. Sci Rep. 2016. PMID: 26728491 Free PMC article.
References
-
- Duncan R. Nanomedicines in action. Pharm. J. 2004;273:485–488.
-
- Royal Society & Royal Academy of Engineering. Nanoscience and Nanotechnologies: Opportunities and Uncertainties. Royal Society; London, UK: 2004.
-
- European Science Foundation. Nanomedicine. An ESF-European Medical Research Councils (EMRC) Forward Look Report. European Science Foundation; Strasbourg, France: 2005.
-
- Malan Y., Loizidou M., Seifalian A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009;30:592–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

