Apolipoprotein mimetic peptides: a new approach for the treatment of asthma
- PMID: 22408624
- PMCID: PMC3297834
- DOI: 10.3389/fphar.2012.00037
Apolipoprotein mimetic peptides: a new approach for the treatment of asthma
Abstract
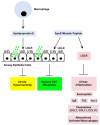
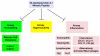
New treatments are needed for severe asthmatics to improve disease control and avoid severe toxicities associated with oral corticosteroids. We have used a murine model of house dust mite (HDM)-induced asthma to identify steroid-unresponsive genes that might represent targets for new therapeutic approaches for severe asthma. This strategy identified apolipoprotein E as a steroid-unresponsive gene with increased mRNA expression in the lungs of HDM-challenged mice. Furthermore, apolipoprotein E functioned as an endogenous negative regulator of airway hyperreactivity and goblet cell hyperplasia in experimental HDM-induced asthma. The ability of apolipoprotein E, which is expressed by lung macrophages, to attenuate AHR, and goblet cell hyperplasia is mediated by low density lipoprotein (LDL) receptors expressed by airway epithelial cells. Consistent with this, administration of an apolipoprotein E mimetic peptide, corresponding to amino acids 130-149 of the LDL receptor-binding domain of the holo-apoE protein, significantly reduced AHR and goblet cell hyperplasia in HDM-challenged apoE(-/-) mice. These findings identified the apolipoprotein E - LDL receptor pathway as a new druggable target for asthma that can be activated by administration of apoE-mimetic peptides. Similarly, apolipoprotein A-I may have therapeutic potential in asthma based upon its anti-inflammatory, anti-oxidative, and anti-fibrotic properties. Furthermore, administration of apolipoprotein A-I mimetic peptides has attenuated airway inflammation, airway remodeling, and airway hyperreactivity in murine models of experimental asthma. Thus, site-directed delivery of inhaled apolipoprotein E or apolipoprotein A-I mimetic peptides may represent novel treatment approaches that can be developed for asthma, including severe disease.
Keywords: apolipoprotein A-I; apolipoprotein A-I mimetic peptide; apolipoprotein E; apolipoprotein E mimetic peptide; asthma.
Figures

References
-
- Amar M. J., D’Souza W., Turner S., Demosky S., Sviridov D., Stonik J., Luchoomun J., Voogt J., Hellerstein M., Remaley A. T. (2010). 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J. Pharmacol. Exp. Ther. 334, 634–64110.1124/jpet.110.167890 - DOI - PMC - PubMed
-
- American Thoracic Society (2000). Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am. J. Respir. Crit. Care Med. 162, 2341–2351 - PubMed
-
- Anantharamaiah G. M., Jones J. L., Brouillette C. G., Schmidt C. F., Chung B. H., Hughes T. A., Bhown A. S., Segrest J. P. (1985). Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J. Biol. Chem. 260, 10248–10255 - PubMed
-
- Anantharamaiah G. M., Mishra V. K., Garber D. W., Datta G., Handattu S. P., Palgunachari M. N., Chaddha M., Navab M., Reddy S. T., Segrest J. P., Fogelman A. M. (2007). Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J. Lipid Res. 48, 1915–192310.1194/jlr.R700010-JLR200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

