Comparing the effects of two inhaled glucocorticoids on allergen-induced bronchoconstriction and markers of systemic effects, a randomised cross-over double-blind study
- PMID: 22409907
- PMCID: PMC3339360
- DOI: 10.1186/2045-7022-1-12
Comparing the effects of two inhaled glucocorticoids on allergen-induced bronchoconstriction and markers of systemic effects, a randomised cross-over double-blind study
Abstract
Background: Inhaled glucocorticoids are efficient in protecting against asthma exacerbations, but methods to compare their efficacy vs systemic effects have only been attempted in larger multi-centre studies. The aim of the current study was therefore to directly compare the effects of two separate inhaled glucocorticoids, mometasone and budesonide, to compare the effects on the early and late asthmatic responses to inhaled allergen in patients with mild allergic asthma, and sputum eosinophils, and to relate the clinical positive effects to any systemic effects observed.
Methods: Twelve patients with documented early and late asthmatic responses (EAR and LAR) to inhaled allergen at a screening visit were randomized in a double-blind fashion to treatment with mometasone (200 μg × 2 or 400 μg × 2), budesonide (400 μg × 2) or placebo in a double-blind crossover fashion for a period of seven days. Challenge with the total allergen dose causing both an EAR and LAR was given on the last day of treatment taken in the morning. Lung function was assessed using FEV1, and systemic glucocorticoid activity was quantified using 24 h urinary cortisol.
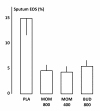
Results: Mometasone and budesonide attenuate both EAR and LAR to allergen to a similar degree. No significant dose-related effects on the lung function parameters were observed. Both treatments reduced the relative amount of sputum eosinophils (%) after allergen. At the dose of 800 μg daily, mometasone reduced 24 h urinary cortisol by approximately 35%. Both drugs were well tolerated.
Conclusions: Mometasone and budesonide are equieffective in reducing early and late asthmatic responses induced by inhaled allergen challenge. Mometasone 800 μg given for seven days partially affects the HPA axis.
Figures



References
-
- de Monchy JG, Aalbers R, Timens W. The clinical relevance of bronchial biopsies in asthma. Respir Med. 1993;87(Suppl B):23–4. - PubMed
-
- Gauvreau GM. et al. The effects of inhaled budesonide on circulating eosinophil progenitors and their expression of cytokines after allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med. 2000;162(6):2139–44. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

