Inhibitory morphogens and monopodial branching of the embryonic chicken lung
- PMID: 22410853
- PMCID: PMC3443594
- DOI: 10.1002/dvdy.23771
Inhibitory morphogens and monopodial branching of the embryonic chicken lung
Abstract
Background: Branching morphogenesis generates a diverse array of epithelial patterns, including dichotomous and monopodial geometries. Dichotomous branching can be instructed by concentration gradients of epithelial-derived inhibitory morphogens, including transforming growth factor-β (TGFβ), which is responsible for ramification of the pubertal mammary gland. Here, we investigated the role of autocrine inhibitory morphogens in monopodial branching morphogenesis of the embryonic chicken lung.
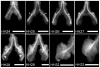
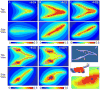
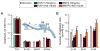
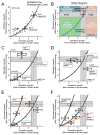
Results: Computational modeling and experiments using cultured organ explants each separately revealed that monopodial branching patterns cannot be specified by a single epithelial-derived autocrine morphogen gradient. Instead, signaling by means of TGFβ1 and bone morphogenetic protein-4 (BMP4) differentially affect the rates of branching and growth of the airways. Allometric analysis revealed that development of the epithelial tree obeys power-law dynamics; TGFβ1 and BMP4 have distinct but reversible effects on the scaling coefficient of the power law.
Conclusions: These data suggest that although autocrine inhibition cannot specify monopodial branching, inhibitory morphogens define the dynamics of lung morphogenesis.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11–18. - PubMed
-
- Becchetti E, Evangelisti R, Stabellini G, Pagliarini A, del Borrello E, Calastrini C, Carinci P. Developmental heterogeneity of mesenchymal glycosaminoglycans (GAG) distribution in chick embryo lung anlagen. Am J Anat. 1988;181:33–42. - PubMed
-
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. - PubMed
-
- Bragg AD, Moses HL, Serra R. Signaling to the epithelium is not sufficient to mediate all of the effects of transforming growth factor beta and bone morphogenetic protein 4 on murine embryonic lung development. Mech Dev. 2001;109:13–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

