Overlapping expression patterns and redundant roles for AP-2 transcription factors in the developing mammalian retina
- PMID: 22411557
- PMCID: PMC3700368
- DOI: 10.1002/dvdy.23762
Overlapping expression patterns and redundant roles for AP-2 transcription factors in the developing mammalian retina
Abstract
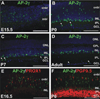
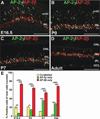
Background: We have previously shown that the transcription factor AP-2α (Tcfap2a) is expressed in postmitotic developing amacrine cells in the mouse retina. Although retina-specific deletion of Tcfap2a did not affect retinogenesis, two other family members, AP-2β and AP-2γ, showed expression patterns similar to AP-2α.
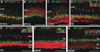
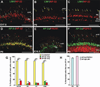
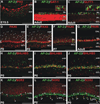
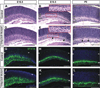
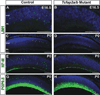
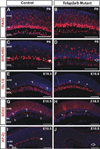
Results: Here we show that, in addition to their highly overlapping expression patterns in amacrine cells, AP-2α and AP-2β are also co-expressed in developing horizontal cells. AP-2γ expression is restricted to amacrine cells, in a subset that is partially distinct from the AP-2α/β-immunopositive population. To address possible redundant roles for AP-2α and AP-2β during retinogenesis, Tcfap2a/b-deficient retinas were examined. These double mutants showed a striking loss of horizontal cells and an altered staining pattern in amacrine cells that were not detected upon deletion of either family member alone.
Conclusions: These studies have uncovered critical roles for AP-2 activity in retinogenesis, delineating the overlapping expression patterns of Tcfap2a, Tcfap2b, and Tcfap2c in the neural retina, and revealing a redundant requirement for Tcfap2a and Tcfap2b in horizontal and amacrine cell development.
Copyright © 2012 Wiley-Liss, Inc.
Figures
References
-
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. - PubMed
-
- Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

