ADAP regulates cell cycle progression of T cells via control of cyclin E and Cdk2 expression through two distinct CARMA1-dependent signaling pathways
- PMID: 22411628
- PMCID: PMC3347422
- DOI: 10.1128/MCB.06541-11
ADAP regulates cell cycle progression of T cells via control of cyclin E and Cdk2 expression through two distinct CARMA1-dependent signaling pathways
Abstract
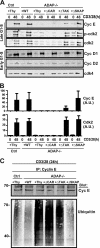
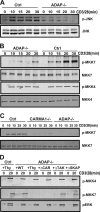
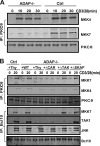
Adhesion and degranulation-promoting adapter protein (ADAP) is a multifunctional scaffold that regulates T cell receptor-mediated activation of integrins via association with the SKAP55 adapter and the NF-κB pathway through interactions with both the CARMA1 adapter and serine/threonine kinase transforming growth factor β-activated kinase 1 (TAK1). ADAP-deficient T cells exhibit impaired proliferation following T cell receptor stimulation, but the contribution of these distinct functions of ADAP to this defect is not known. We demonstrate that loss of ADAP results in a G₁-S transition block in cell cycle progression following T cell activation due to impaired accumulation of cyclin-dependent kinase 2 (Cdk2) and cyclin E. The CARMA1-binding site in ADAP is critical for mitogen-activated protein (MAP) kinase kinase 7 (MKK7) phosphorylation and recruitment to the protein kinase C θ (PKCθ) signalosome and subsequent c-Jun kinase (JNK)-mediated Cdk2 induction. Cyclin E expression following T cell receptor stimulation of ADAP-deficient T cells is transient and associated with enhanced cyclin E ubiquitination. Both the CARMA1- and TAK1-binding sites in ADAP are critical for restraining cyclin E ubiquitination and turnover independently of ADAP-dependent JNK activation. T cell receptor-mediated proliferation was most dramatically impaired by the loss of ADAP interactions with CARMA1 or TAK1 rather than SKAP55. Thus, ADAP coordinates distinct CARMA1-dependent control of key cell cycle proteins in T cells.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
