Lateral mobility of individual integrin nanoclusters orchestrates the onset for leukocyte adhesion
- PMID: 22411821
- PMCID: PMC3323969
- DOI: 10.1073/pnas.1116425109
Lateral mobility of individual integrin nanoclusters orchestrates the onset for leukocyte adhesion
Abstract
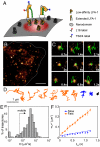
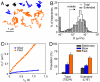
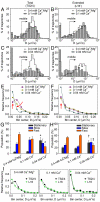
Integrins are cell membrane adhesion receptors involved in morphogenesis, immunity, tissue healing, and metastasis. A central, yet unresolved question regarding the function of integrins is how these receptors regulate both their conformation and dynamic nanoscale organization on the membrane to generate adhesion-competent microclusters upon ligand binding. Here we exploit the high spatial (nanometer) accuracy and temporal resolution of single-dye tracking to dissect the relationship between conformational state, lateral mobility, and microclustering of the integrin receptor lymphocyte function-associated antigen 1 (LFA-1) expressed on immune cells. We recently showed that in quiescent monocytes, LFA-1 preorganizes in nanoclusters proximal to nanoscale raft components. We now show that these nanoclusters are primarily mobile on the cell surface with a small (ca. 5%) subset of conformational-active LFA-1 nanoclusters preanchored to the cytoskeleton. Lateral mobility resulted crucial for the formation of microclusters upon ligand binding and for stable adhesion under shear flow. Activation of high-affinity LFA-1 by extracellular Ca(2+) resulted in an eightfold increase on the percentage of immobile nanoclusters and cytoskeleton anchorage. Although having the ability to bind to their ligands, these active nanoclusters failed to support firm adhesion in static and low shear-flow conditions because mobility and clustering capacity were highly compromised. Altogether, our work demonstrates an intricate coupling between conformation and lateral diffusion of LFA-1 and further underscores the crucial role of mobility for the onset of LFA-1 mediated leukocyte adhesion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
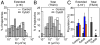

References
-
- Takagi J, et al. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–611. - PubMed
-
- Takagi J, Springer TA. Integrin activation and structural rearrangement. Immunol Rev. 2002;186:141–163. - PubMed
-
- van Kooyk Y, van Vliet SJ, Figdor CG. The actin cytoskeleton regulates LFA-1 ligand binding through avidity rather than affinity changes. J Biol Chem. 1999;274:26869–26877. - PubMed
-
- Katagiri K, et al. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

