Obatoclax interacts synergistically with the irreversible proteasome inhibitor carfilzomib in GC- and ABC-DLBCL cells in vitro and in vivo
- PMID: 22411899
- PMCID: PMC3601737
- DOI: 10.1158/1535-7163.MCT-12-0021
Obatoclax interacts synergistically with the irreversible proteasome inhibitor carfilzomib in GC- and ABC-DLBCL cells in vitro and in vivo
Retraction in
-
Retraction: Obatoclax Interacts Synergistically with the Irreversible Proteasome Inhibitor Carfilzomib in GC- and ABC-DLBCL Cells In Vitro and In Vivo.Mol Cancer Ther. 2019 Jun;18(6):1180. doi: 10.1158/1535-7163.MCT-19-0470. Mol Cancer Ther. 2019. PMID: 31160510 Free PMC article. No abstract available.
Abstract
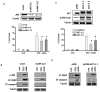
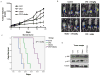
Interactions between the irreversible proteasome inhibitor carfilzomib and the pan-BH3 mimetic obatoclax were examined in germinal center (GC)- and activated B-cell-diffuse large B-cell lymphoma (ABC-DLBCL) cells. Cotreatment with minimally toxic concentrations of carfilzomib (i.e., 2-6 nmol/L) and subtoxic concentrations of obatoclax (0.05-2.0 μmol/L) synergistically increased apoptosis in multiple DLBCL cell lines and increased lethality toward primary human DLBCL but not normal CD34(+) cells. Synergistic interactions were associated with sharp increases in caspase-3 activation, PARP cleavage, p-JNK induction, upregulation of Noxa, and AKT dephosphorylation. Combined treatment also diminished carfilzomib-mediated Mcl-1 upregulation whereas immunoprecipitation analysis revealed reduced associations between Bak and Mcl-1/Bcl-xL and Bim and Mcl-1. The carfilzomib/obatoclax regimen triggered translocation, conformational change, and dimerization of Bax and activation of Bak. Genetic interruption of c-jun-NH(2)-kinase (JNK) and Noxa by short hairpin RNA knockdown, ectopic Mcl-1 expression, or enforced activation of AKT significantly attenuated carfilzomib/obatoclax-mediated apoptosis. Notably, coadministration of carfilzomib/obatoclax sharply increased apoptosis in multiple bortezomib-resistant DLBCL models. Finally, in vivo administration of carfilzomib and obatoclax to mice inoculated with SUDHL4 cells substantially suppressed tumor growth, activated JNK, inactivated AKT, and increased survival compared with the effects of single-agent treatment. Together, these findings argue that a strategy combining carfilzomib and obatoclax warrants attention in DLBCL.
©2012 AACR
Conflict of interest statement
Potential conflict of interest: No conflict of interest to report
Figures
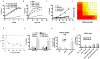



Comment in
-
Findings of Research Misconduct.NIH Guide Grants Contracts (Bethesda). 2015 Dec 18:NOT-OD-16-040. NIH Guide Grants Contracts (Bethesda). 2015. PMID: 26693581 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2015 Dec 10;80(237):76703-76704. Fed Regist. 2015. PMID: 27737268 Free PMC article. No abstract available.
References
-
- Coiffier B. Immunochemotherapy: the new standard in aggressive non-Hodgkin’s lymphoma in the elderly. Semin Oncol. 2003;30:21–7. - PubMed
-
- Coiffier B. Rituximab in combination with CHOP improves survival in elderly patients with aggressive non-Hodgkin’s lymphoma. Semin Oncol. 2002;29:18–22. - PubMed
-
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17. - PubMed
-
- Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de VS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50CA142509/CA/NCI NIH HHS/United States
- CA63753/CA/NCI NIH HHS/United States
- R01 CA100866/CA/NCI NIH HHS/United States
- R01 CA063753/CA/NCI NIH HHS/United States
- R01 CA093738/CA/NCI NIH HHS/United States
- CA100866/CA/NCI NIH HHS/United States
- P50 CA130805/CA/NCI NIH HHS/United States
- R01 CA141703/CA/NCI NIH HHS/United States
- CA93738/CA/NCI NIH HHS/United States
- R01 DK052825/DK/NIDDK NIH HHS/United States
- R01 CA150214/CA/NCI NIH HHS/United States
- 1P50 CA130805/CA/NCI NIH HHS/United States
- P50 CA142509/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

