Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100
- PMID: 22411952
- PMCID: PMC4281708
- DOI: 10.1158/0008-5472.CAN-11-3980
Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100
Abstract
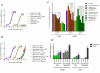
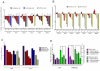
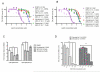
Prostate cancer progression can be associated with androgen receptor (AR) mutations acquired following treatment with castration and/or an antiandrogen. Abiraterone, a rationally designed inhibitor of CYP17A1 recently approved for the treatment of docetaxel-treated castration-resistant prostate cancer (CRPC), is often effective, but requires coadministration with glucocorticoids to curtail side effects. Here, we hypothesized that progressive disease on abiraterone may occur secondary to glucocorticoid-induced activation of mutated AR. We found that prednisolone plasma levels in patients with CRPC were sufficiently high to activate mutant AR. Mineralocorticoid receptor antagonists, such as spironolactone and eplerenone that are used to treat side effects related to mineralocorticoid excess, can also bind to and activate signaling through wild-type or mutant AR. Abiraterone inhibited in vitro proliferation and AR-regulated gene expression of AR-positive prostate cancer cells, which could be explained by AR antagonism in addition to inhibition of steroidogenesis. In fact, activation of mutant AR by eplerenone was inhibited by MDV3100, bicalutamide, or greater concentrations of abiraterone. Therefore, an increase in abiraterone exposure could reverse resistance secondary to activation of AR by residual ligands or coadministered drugs. Together, our findings provide a strong rationale for clinical evaluation of combined CYP17A1 inhibition and AR antagonism.
©2012 AACR
Figures

Comment in
-
Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100.Cancer Res. 2013 May 1;73(9):2926. doi: 10.1158/0008-5472.CAN-12-2910. Epub 2013 Apr 22. Cancer Res. 2013. PMID: 23610449 No abstract available.
References
-
- Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. - PubMed
-
- Scher H, Fizazi K, Saad F, Taplin ME, Sternberg C, miller k, et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. GU ASCO conference; San Francisco. 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

