Chaperone-usher pathways: diversity and pilus assembly mechanism
- PMID: 22411982
- PMCID: PMC3297437
- DOI: 10.1098/rstb.2011.0206
Chaperone-usher pathways: diversity and pilus assembly mechanism
Abstract
Up to eight different types of secretion systems, and several more subtypes, have been described in Gram-negative bacteria. Here, we focus on the diversity and assembly mechanism of one of the best-studied secretion systems, the widespread chaperone-usher pathway known to assemble and secrete adhesive surface structures, called pili or fimbriae, which play essential roles in targeting bacterial pathogens to the host.
Figures

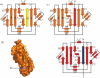

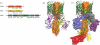
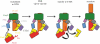
References
-
- Houwink A. L., van Iterson W. 1950. Electron microscopical observations on bacterial cytology; a study on flagellation. Biochim. Biophys. Acta 5, 10–4410.1016/0006-3002(50)90144-2 (doi:10.1016/0006-3002(50)90144-2) - DOI - DOI - PubMed
-
- Duguid J. P., Smith I. W., Dempster G., Edmunds P. N. 1955. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli . J. Pathol. Bacteriol. 70, 335–34810.1002/path.1700700210 (doi:10.1002/path.1700700210) - DOI - DOI - PubMed
-
- Brinton C. C. 1959. Non-flagellar appendages of bacteria. Nature 183, 782–78610.1038/183782a0 (doi:10.1038/183782a0) - DOI - DOI - PubMed
-
- Brinton C. C. 1965. The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in Gram negative bacteria. Trans. N. Y. Acad. Sci. 27, 1003–1054 - PubMed
-
- Duguid J. P., Anderson E. S., Campbell I. 1966. Fimbriae and adhesive properties in Salmonellae. J. Pathol. Bacteriol. 92, 107–13810.1002/path.1700920113 (doi:10.1002/path.1700920113) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

