The blueprint of the type-3 injectisome
- PMID: 22411984
- PMCID: PMC3297436
- DOI: 10.1098/rstb.2011.0205
The blueprint of the type-3 injectisome
Abstract
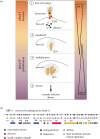
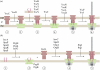
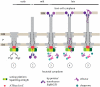
Type-3 secretion systems are sophisticated syringe-like nanomachines present in many animal and plant Gram-negative pathogens. They are capable of translocating an arsenal of specific bacterial toxins (effector proteins) from the prokaryotic cytoplasm across the three biological membranes directly into the eukaryotic cytosol, some of which modulate host cell mechanisms for the benefit of the pathogen. They populate a particular biological niche, which is maintained by specific, pathogen-dependent effectors. In contrast, the needle complex, which is the central component of this specialized protein delivery machine, is structurally well-conserved. It is a large supramolecular cylindrical structure composed of multiple copies of a relatively small subset of proteins, is embedded in the bacterial membranes and protrudes from the pathogen's surface with a needle filament. A central channel traverses the entire needle complex, and serves as a hollow conduit for proteins destined to travel this secretion pathway. In the past few years, there has been a tremendous increase in an understanding on both the structural and the mechanistic level. This review will thus focus on new insights of this remarkable molecular machine.
Figures



References
-
- Coburn B., Sekirov I., Finlay B. B. 2007. Type III secretion systems and disease. Clin. Microbiol. Rev. 20, 535–54910.1128/CMR.00013-07 (doi:10.1128/CMR.00013-07) - DOI - DOI - PMC - PubMed
-
- Galan J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17, 53–8610.1146/annurev.cellbio.17.1.53 (doi:10.1146/annurev.cellbio.17.1.53) - DOI - DOI - PubMed
-
- Akopyan K., Edgren T., Wang-Edgren H., Rosqvist R., Fahlgren A., Wolf-Watz H., Fallman M. 2011. Translocation of surface-localized effectors in type III secretion. Proc. Natl Acad. Sci. USA 108, 1639–164410.1073/pnas.1013888108 (doi:10.1073/pnas.1013888108) - DOI - DOI - PMC - PubMed
-
- Galan J. E., Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–57310.1038/nature05272 (doi:10.1038/nature05272) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
