Mononuclear iron enzymes are primary targets of hydrogen peroxide stress
- PMID: 22411989
- PMCID: PMC3346116
- DOI: 10.1074/jbc.M111.330365
Mononuclear iron enzymes are primary targets of hydrogen peroxide stress
Abstract
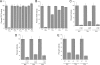
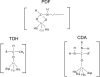
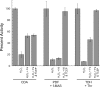
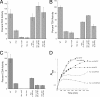
This study tested whether nonredox metalloenzymes are commonly charged with iron in vivo and are primary targets of oxidative stress because of it. Indeed, three sample mononuclear enzymes, peptide deformylase, threonine dehydrogenase, and cytosine deaminase, were rapidly damaged by micromolar hydrogen peroxide in vitro and in live Escherichia coli. The first two enzymes use a cysteine residue to coordinate the catalytic metal atom; it was quantitatively oxidized by the radical generated by the Fenton reaction. Because oxidized cysteine can be repaired by cellular reductants, the effect was to avoid irreversible damage to other active-site residues. Nevertheless, protracted H(2)O(2) exposure gradually inactivated these enzymes, consistent with the overoxidation of the cysteine residue to sulfinic or sulfonic forms. During H(2)O(2) stress, E. coli defended all three proteins by inducing MntH, a manganese importer, and Dps, an iron-sequestration protein. These proteins appeared to collaborate in replacing the iron atom with nonoxidizable manganese. The implication is that mononuclear metalloproteins are common targets of H(2)O(2) and that both structural and metabolic arrangements exist to protect them.
Figures

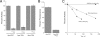


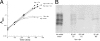
References
-
- Delaunay A., Pflieger D., Barrault M. B., Vinh J., Toledano M. B. (2002) A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111, 471–481 - PubMed
-
- Choi H., Kim S., Mukhopadhyay P., Cho S., Woo J., Storz G., Ryu S. (2001) Structural basis of the redox switch in the OxyR transcription factor. Cell 105, 103–113 - PubMed
-
- Lee J. W., Helmann J. D. (2006) The PerR transcription factor senses H2O2 by metal-catalyzed histidine oxidation. Nature 440, 363–367 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

