A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells
- PMID: 22412018
- PMCID: PMC3308701
- DOI: 10.1083/jcb.201112098
A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells
Abstract
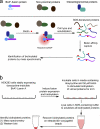
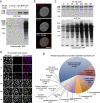
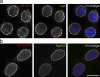
We have developed a new technique for proximity-dependent labeling of proteins in eukaryotic cells. Named BioID for proximity-dependent biotin identification, this approach is based on fusion of a promiscuous Escherichia coli biotin protein ligase to a targeting protein. BioID features proximity-dependent biotinylation of proteins that are near-neighbors of the fusion protein. Biotinylated proteins may be isolated by affinity capture and identified by mass spectrometry. We apply BioID to lamin-A (LaA), a well-characterized intermediate filament protein that is a constituent of the nuclear lamina, an important structural element of the nuclear envelope (NE). We identify multiple proteins that associate with and/or are proximate to LaA in vivo. The most abundant of these include known interactors of LaA that are localized to the NE, as well as a new NE-associated protein named SLAP75. Our results suggest BioID is a useful and generally applicable method to screen for both interacting and neighboring proteins in their native cellular environment.
Figures

References
-
- Bodoor K., Shaikh S., Salina D., Raharjo W.H., Bastos R., Lohka M., Burke B. 1999. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J. Cell Sci. 112:2253–2264 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

